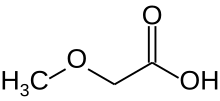
Chemistry:Methoxyacetic acid
| |
| Names | |
|---|---|
| IUPAC name
2-Methoxyacetic acid
| |
| Other names
2-Methoxyacetic acid
Methyl glycolic acid | |
| Identifiers | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| C 3H 6O 3 | |
| Molar mass | 90.08 g/mol−1 |
| Appearance | Clear, colorless, viscous liquid with a pungent odor |
| Density | 1.1768 g/cm−3 |
| Melting point | 7–9 °C (45–48 °F; 280–282 K) |
| Boiling point | 202–204 °C (396–399 °F; 475–477 K) |
| Soluble in water, ethanol, and diethyl ether | |
| Vapor pressure | 1.8 mbar (20 °C) 4.8 mbar (50 °C) |
| Acidity (pKa) | 3.57 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methoxyacetic acid is a derivative of acetic acid in which a hydrogen atom of the methyl group is replaced by a methoxy group. As indicated by the synonym methyl glycolic acid, and as the simplest ether carboxylic acid, methoxyacetic acid can be understood as a methyl ether of glycolic acid.
Due to its considerable reprotoxic potential, methoxyacetic acid has been adopted into the list of SVHC substances (substances of very high concern)[1] and is only registered as an intermediate product for industrial purposes under strictly controlled conditions.
For consumer applications, such as for the cleaning and decalcification of surfaces, the substance must be substituted by safe alternatives.
Production
With twice the molar amount of sodium methoxide in methanol, the conversion of monochloroacetic acid yields, after acidification of dry hydrogen chloride gas and vacuum distillation, methoxyacetic acid in amounts of about 90%.[2]
The route of synthesis is inefficient as it requires relatively expensive raw materials and large amounts of the intermediate product sodium chloride.
When methyl glycol is oxidized with concentrated nitric acid – even in the presence of vanadium(V) oxide – methoxyacetic acid is produced at a rate of about 85%.[3]
A disadvantage of the reaction using (excessively) hot nitric acid is the formation of nitrous gases, which – much like excessive nitric acid via the addition of urea and formaldehyde – must be avoided.
The most common procedure by industrial standards is the oxidation of methyl glycol with air or oxygen in the presence of platinum catalysts in a relatively high (10–30%) aqueous solution at a pH value of ≤ 7 and temperatures around 50 °C, producing amounts of up to 95% and space-time yields of 150 g · 1−1h−1.[4]
In both humans and animals, 2-methoxyacetic acid forms via the rapid oxidation of 2-methoxyethanol (methyl glycol)[5] via alcohol dehydrogenases.
Properties
Methoxyacetic acid is a clear, colorless, viscous, and corrosive liquid with a pungent odor which, at 7 °C, freezes to a mass similar to glacial acetic acid. Due to the low solvation energy of its methoxy group, methoxyacetic acid, with a pKa value of 3.57, is more acidic than acetic acid (pKa 4.757) and glycolic acid (pKa 3.832).[6]
Ultra-pure methoxyacetic acid (purity of 99.8%, freezing point of 8.4 °C) can be obtained via the multistep crystallization of the raw distillate, which is free of acid contaminations.[7]
Applications
Due to its reprotoxic properties, earlier consumer and industrial applications of methoxyacetic acid as a disinfectant, biocide, or as a cleaner for the decalcification of surfaces are now obsolete. The same is true for substances such as the solvent 2-methoxyethanol or the PVC plasticizer bis(2-methoxyethyl) phthalate, which are metabolized to methoxyacetic acid in the body.
As a molecular component of multiple iodized aromatic compounds, methoxyacetic acid was once used in X-ray contrast agents.[8]
In laboratory tests, methoxyacetic acid inhibits the growth of tumor cells.[9]
References
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.009.904.
- ↑ "Espacenet – search results". https://worldwide.espacenet.com/patent/search/family/010977901/publication/US4968840A?q=pn=US4968840.
- ↑ "Espacenet – search results". https://worldwide.espacenet.com/patent/search/family/004248134/publication/DE2832949A1?q=pn=DE2832949.
- ↑ "Espacenet – search results". https://worldwide.espacenet.com/patent/search/family/006080251/publication/DE2936123A1?q=pn=DE2936123A1.
- ↑ Mebus, C.A.; Welsch, F. (1989). "The possible role of one-carbon moieties in 2-methoxyethanol and 2-methoxyacetic acid-induced developmental toxicity" (in en). Toxicology and Applied Pharmacology 99 (1): 98–109. doi:10.1016/0041-008X(89)90115-4. PMID 2471293. https://linkinghub.elsevier.com/retrieve/pii/0041008X89901154.
- ↑ King, Edward J. (1960). "The Thermodynamics of Ionization of Amino Acids. V. The Ionization Constants of 3-Methoxy-DL-alanine (O-Methylserine) and Methoxyacetic Acid 1" (in en). Journal of the American Chemical Society 82 (14): 3575–3578. doi:10.1021/ja01499a025. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01499a025.
- ↑ "Espacenet – search results". https://worldwide.espacenet.com/patent/search/family/006217320/publication/DE3345807A1?q=pn=DE3345807A1.
- ↑ "Espacenet – search results". https://worldwide.espacenet.com/patent/search/family/006065014/publication/US4364921A?q=pn=US4364921.
- ↑ Parajuli, Keshab R.; Zhang, Qiuyang; Liu, Sen; Patel, Neil K.; Lu, Hua; Zeng, Shelya X.; Wang, Guangdi; Zhang, Changde et al. (2014). "Methoxyacetic acid suppresses prostate cancer cell growth by inducing growth arrest and apoptosis". American Journal of Clinical and Experimental Urology 2 (4): 300–312. ISSN 2330-1910. PMID 25606576.

