Chemistry:Methyldiborane
| |
| Names | |
|---|---|
| IUPAC name
Methyldiborane
| |
| Other names
monomethyldiborane
methylated diborane boraethane | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| Properties | |
| CH3BH3BH2 | |
| Molar mass | 41.70 g mol−1 |
| Appearance | Colorless gas |
| Density | 0.546 at -126° |
| Boiling point | −43 °C (−45 °F; 230 K) |
| Related compounds | |
| dimethyldiborane trimethyldiborane tetramethyldiborane trimethylborane ethyldiborane | |
Related compounds
|
Diborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
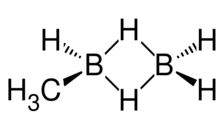
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3).[4] Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.[5]
The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.[6][7]
Formation
Methylboranes are formed by the reaction of diborane and trimethylborane. This reaction produces four different substitution of methyl with hydrogen on diborane. Produced is 1-methyldiborane, 1,1-dimethyldborane, 1,1,2-trimethyldiborane and 1,1,2,2-tetramethyldiborane.[5] The reaction is complex. At 0 °C when diborane is in excess, monomethyldiborane is initially produced, coming to a steady but low level, and 1,1-dimethyldiborane level increases over a long time, until all trimethylborane is consumed. Monomethyldiborane ends up at equilibrium with a mixture of diborane and dimethyldiborane. At 0° the equilibrium constant for 2B2H5Me ←→ B2H6 + (BH2Me)2 is around 0.07, so monomethyldiborane will typically be the majority of the mixture, but there will still be a significant amount of diborane and dimethyldiborane present.[8] Monomethyldiborane yield is best with a ratio of 4 of diborane to 1 of trimethylborane.[9] The yield of trimethyldiborane is maximised with ratio of 1 of diborane to 3 of trimethylborane.[9]
When methyllithium reacts with diborane, monomethyldiborane is produced in about a 20% yield.[10]
Tetramethyl lead can react with diborane in a 1,2-dimethoxyethane solvent at room temperature to make a range of methyl substituted diboranes, ending up at trimethylborane, but including 1,1-di, tridiborane. The other outputs of the reaction are hydrogen gas and lead metal.[11]
Other methods to form methyldiboranes include reacting hydrogen with trimethylborane between 80 and 200 °C under pressure, or reacting a metal borohydride with trimethylborane in the presence of hydrogen chloride, aluminium chloride or boron trichloride. If the borohydride is sodium borohydride, then methane is a side product. If the metal is lithium then no methane is produced.[6] dimethylchloroborane and methyldichloroborane are also produced as gaseous products.[6]
When Cp2Zr(CH3)2 reacts with borane dissolved in tetrahydrofuran, a borohydro group inserts into the zirconium carbon bond, and methyl diboranes are produced.[12]
When trimethylgallium reacts with diborane at -45°, methyldiborane is produced along with dimethylgallium borohydride.
- 2(CH3)3Ga + B2H6 → (CH3)2GaBH4 + CH3B2H5.[13]
At room temperature trimethylgallium reacts with diborane to make a volatile substance that decomposes to gallium metal along with methyldiborane.
- (CH3)3Ga + 3B2H6 → Ga + 3CH3B2H5 +1.5H2.[13]
Properties
The compound boils at −43 °C.[14] Methyldiborane liquid has a density of 0.546 g/ml at −126°[15] At −78.5 the vapour pressure is 55 torr.[15]
Methyldiborane HCH3BH2BH2 has one methyl group and a hydrogen on a boron atom. The other boron atom is only bound to hydrogen atoms. A bridge of two hydrogen atoms links the boron atoms together. The methyldiborane molecule has the following measurements: B1 is the boron atom not attached to the methyl group and B2 is the boron atom that has methyl group attached, and Hμ is one of the bridge hydrogen atoms between the boron atoms. The distance between boron atoms is 1.82 Å, Distance between boron atoms and bridging hydrogen atoms is 1.34 Å. Distances to non bridging hydrogens from B1 are 1.195 and 1.187 Å. B2 distance to non bridging hydrogen is 1.2 Å. Distance between two bridging hydrogen atoms is 1.96 Å. The carbon to boron bond is 1.49 Å long. The angle subtended from the bridging hydrogens to the boron to boron axis is 47°. The angle of carbon to the boron-boron axis is 120°. The dipole moment of methyldiborane is 0.56 D.[16] It has a vapour pressure of 61 mm Hg at −77.2 °C.[9] The predicted heat of formation for the liquid is ΔH0f=−14 kcal/mol, and for the gas −9 kcal/mol.[17]
A gas chromatograph can be used to determine the amounts of the methyl boranes in a mixture. The order they pass through are diborane, monomethyldiborane, trimethylborane, 1,1-dimethyldiborane, 1,2-dimethyldiborane, trimethyldiborane, and last tetramethyldiborane.[18]
The nuclear resonance shift for the bridge hydrogen is 10.09 ppm, compared to 10.49 for diborane.[19]
Reactions
At -78.5 °C methyldiborane disproportionates slowly first to diborane and 1,1-dimethyldiborane.[15] In solution methylborane is more stable against disproportionation than dimethylborane.[20]
- 2MeB2H5 ⇌ 1,1-Me2B2H4 + B2H6 K=2.8 Me=CH3.[21]
By reacting methyldiborane with ether, dimethylether borine is formed (CH3)2O.BH3 leaving methylborane which rapidly dimerises to 1,2-dimethyldiborane.[5][22]
Methyldiborane is hydrolyzed in water to methylboronic acid CH3B(OH)2.[5] Methyldiborane reacts with trimethylamine to yield solid derivatives trimethylamine-methylborane (CH3)3N—BHCH3 and trimethylamine-borane (CH3)3N—BH3.[5]
Methyldiborane is pyrophoric, spontaneously inflaming when exposed to air.[15]
When methyldiborane is oxidised around 150 °C a substance 2-methyl-1,3,4-trioxadiboralane is produced. This is a ring of three oxygen atoms and two boron atoms, with methyl attached to one boron atom. At the same time dimethyltrioxadiboralane and trimethylboroxine are also formed, and also hydrocarbons, diborane, hydrogen, and dimethoxyborane (dimethyl methylboronic ester).[23]
When methyldiborane, or dimethyldiborane is combined with ammonia, aminodimethylborine (NH2BMe2) is formed and on heating around 180 °C B-methyl borazoles are produced. These borazoles can have zero, one, two or three methyl groups substituted on the boron atoms (B3N3H6, MeB3N3H5, Me2B3N3H4 or Me3B3N3H3).[24][25]
A specific way to make 1,2-dimethyldiborane is to react methyldiborane with a sufficient amount of a Lewis base. This will strip off borane to combine with the Lewis base, and let two methyl borane molecules dimerise.[26]
Methyldiborane can methylate tetraborane.
- CH3B2H5 + B4H10 → 2-CH3B4H9 + B2H6[27]
Related
Bis(trimethylphosphine) methyldiborane is an adduct of methyldiborane formed when methylpentaborane (1-CH3B5H8 or 2-CH3B5H8) is reacted with trimethylphosphine.[28]
References
- ↑ Jane E. Macintyre, ed (1994-11-10). Dictionary of Organometallic Compounds. CRC Press. p. 463. ISBN 978-0-412-43060-2. https://books.google.com/books?id=6F4MeeopZWQC&pg=PA463.
- ↑ http://cactus.nci.nih.gov/chemical/structure/C%5BBH%5D1H%5BBH2%5DH1/stdinchi [bare URL plain text file]
- ↑ http://cactus.nci.nih.gov/chemical/structure/C%5BBH%5D1H%5BBH2%5DH1/stdinchikey [bare URL plain text file]
- ↑ Srebnik, Morris; Cole, Thomas E.; Brown, Herbert C. (January 1987). "Methylborane - a remarkable unhindered monoalkylborane which achieves the controlled sequential hydroboration of representative alkenes". Tetrahedron Letters 28 (33): 3771–3774. doi:10.1016/s0040-4039(00)96380-9.
- ↑ 5.0 5.1 5.2 5.3 5.4 Bell, R. P.; Emeléus, H. J. (1948). "The boron hydrides and related compounds". Quarterly Reviews, Chemical Society 2 (2): 132. doi:10.1039/QR9480200132. (Subscription content?)
- ↑ 6.0 6.1 6.2 Long, L. H.; Wallbridge, M. G. H. (1965). "646. The chemistry of boron. Part VI. New preparative methods and decomposition studies relating to methyldiboranes". Journal of the Chemical Society: 3513–3520. doi:10.1039/JR9650003513. (Subscription content?)
- ↑ Schlesinger, H. I.; Walker, A. O. (April 1935). "Hydrides of Boron. IV. The Methyl Derivatives of Diborane". Journal of the American Chemical Society 57 (4): 621–625. doi:10.1021/ja01307a009.
- ↑ van Aalten, Lloyd; Seely, G. R.; Oliver, Juhn; Ritter, D. M. (1 June 1961). Kinetics and Equilibria in the Alkylation of Diborane Preliminary Report. Advances in Chemistry. 32. American Chemical Society. pp. 107–114. doi:10.1021/ba-1961-0032.ch012. ISBN 978-0-8412-0033-3. https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/4264-BORAX-TO-BORANES3db1.pdf?topic=2297.0.
- ↑ 9.0 9.1 9.2 Carpenter, J.H.; Jones, W.J.; Jotham, R.W.; Long, L.H. (September 1971). "The Raman spectra of the methyldiboranes—II Monomethyldiborane and trimethyldiborane, and characteristic frequencies of the methyldiboranes". Spectrochimica Acta Part A: Molecular Spectroscopy 27 (9): 1721–1734. doi:10.1016/0584-8539(71)80227-1. Bibcode: 1971AcSpA..27.1721C.
- ↑ Massey, A. G. (1979). "Chapter 2. The typical elements. Part II: Group III". Annual Reports on the Progress of Chemistry, Section A 76: 13–15. doi:10.1039/IC9797600013. (Subscription content?)
- ↑ Holliday, A.K.; N. Jessop, G. (November 1967). "The reaction of tetramethyllead with diborane". Journal of Organometallic Chemistry 10 (2): 291–293. doi:10.1016/s0022-328x(00)93089-4.
- ↑ Marsella, John A.; Caulton, Kenneth G. (May 1982). "Dealkylation of zirconium(IV) by borane: the intimate mechanism of an alkyl transfer reaction". Journal of the American Chemical Society 104 (9): 2361–2365. doi:10.1021/ja00373a005.
- ↑ 13.0 13.1 Schlesinger, H. I.; Brown, Herbert C (September 1943). "The Borohydrides of Gallium". Journal of the American Chemical Society 65 (9): 1787. doi:10.1021/ja01249a512. http://pubs.acs.org/doi/abs/10.1021/ja01249a035?journalCode=jacsat. Retrieved 17 August 2015.
- ↑ "Thermal reaction of diborane with trimethylborane". National Advisory Committee for Aeronautics. 4 September 1958. https://digital.library.unt.edu/ark:/67531/metadc64257/m1/1/.
- ↑ 15.0 15.1 15.2 15.3 Bunting, Roger K. (22 Sep 2009). "55 1-Methyldiborane". in Duward F. Shriver. Inorganic Syntheses, Volume 19. John Wiley and Sons. pp. 237–238. ISBN 978-0-471-04542-7. https://books.google.com/books?id=Tk3HUFj9XnkC&pg=PA237.
- ↑ Chiu, C. W.; Burg, A. B.; Beaudet, R. A. (March 1982). "Molecular structure determination of methyldiborane". Inorganic Chemistry 21 (3): 1204–1208. doi:10.1021/ic00133a064.
- ↑ Altschuller, Aubrey P. (4 October 1955). "Calculated Heats of Formation and Combustion of Boron Compounds (Boron, Hydrogen, Carbon, Silicon)". Cleveland, Ohio: National Advisory Committee for Aeronautics. p. 22. http://naca.central.cranfield.ac.uk/reports/1955/naca-rm-e55g26.pdf.
- ↑ Seely, G. R.; Oliver, J. P.; Ritter, D. M. (December 1959). "Gas-Liquid Chromatographic Analysis of Mixtures Containing Methyldiboranes". Analytical Chemistry 31 (12): 1993–1995. doi:10.1021/ac60156a032.
- ↑ Leach, John B.; Ungermann, Charles B.; Onak, Thomas P. (January 1972). "Proton magnetic resonance studies on methyl and chloro substituted diboranes". Journal of Magnetic Resonance 6 (1): 74–83. doi:10.1016/0022-2364(72)90088-1. Bibcode: 1972JMagR...6...74L.
- ↑ Brown, Herbert C.; Cole, Thomas E.; Srebnik, Morris; Kim, Kee Won (December 1986). "Hydroboration. 79. Preparation and properties of methylborane and dimethylborane and their characteristics as hydroborating agents. Synthesis of tertiary alcohols containing methyl groups via hydroboration". The Journal of Organic Chemistry 51 (25): 4925–4930. doi:10.1021/jo00375a031.
- ↑ Onak, Thomas (1 January 1966). "Carboranes and Organo-Substituted Boron Hydrides". in Stone, F. G. A.; West, Robert. Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. ISBN 978-0-08-058004-3. https://books.google.com/books?id=AHEPoIx22iwC&pg=PA266. Retrieved 19 August 2015.
- ↑ Adams, Roy M. (September 1959). "Organoboron Compounds". Metal-Organic Compounds. Advances in Chemistry. 23. p. 92. doi:10.1021/ba-1959-0023.ch010. ISBN 978-0-8412-0024-1. https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/4262-AIC-023-METAL-ORGANIC-COMPOUNDS-13db1.pdf?topic=2297.0. Retrieved 17 August 2015.
- ↑ Barton, Lawrence; Crump, John M.; Wheatley, Jeffrey B. (June 1974). "Trioxadiborolanes from the oxidation of methyldiborane". Journal of Organometallic Chemistry 72 (1): C1–C3. doi:10.1016/s0022-328x(00)82027-6.
- ↑ Sheldon, J. C.; Smith, B. C. (1960). "The borazoles". Quarterly Reviews, Chemical Society 14 (2): 202. doi:10.1039/QR9601400200.
- ↑ Schlesinger, H. I.; Horvitz, Leo; Burg, A. B. (March 1936). "Hydrides of Boron. VI. The Action of Ammonia on the Methyl Diboranes". Journal of the American Chemical Society 58 (3): 409–414. doi:10.1021/ja01294a008.
- ↑ Wade, Kenneth (1971). "The General Chemistry of the Boron Hydrides". Electron Deficient Compounds. Thomas Nelson. pp. 91–92. ISBN 978-1-4684-6056-8. https://books.google.com/books?id=7oXgBwAAQBAJ&pg=PA92. Retrieved 17 August 2015.
- ↑ Onak, Thomas (1975). Organoborane chemistry. New York: Academic Press. pp. 193–194. ISBN 978-0-12-526550-8. https://books.google.com/books?id=AKl_M5FrL_sC&pg=PA193. Retrieved 17 August 2015.
- ↑ Kameda, Mitsuaki; Driscoll, Jerry A.; Kodama, Goji (September 1990). "Formation and reactions of bis(trimethylphosphine)-methyldiborane(4)". Inorganic Chemistry 29 (19): 3791–3795. doi:10.1021/ic00344a028.
Extra reading
- Carpenter, J. H.; Jones, W. J.; Jotham, R. W.; Long, L. H. (1968). "Laser-source Raman spectroscopy and the Raman spectra of the methyldiboranes". Chemical Communications (15): 881. doi:10.1039/C19680000881.
- Lehmann, Walter J.; Wilson, Charles O.; Shapiro, I. (1960). "Infrared Spectra of Alkyldiboranes. I. Monomethyldiboranes". The Journal of Chemical Physics 32 (4): 1088. doi:10.1063/1.1730853. Bibcode: 1960JChPh..32.1088L.
- Crompton, T. R. (6 December 2012). Gas Chromatography of Organometallic Compounds. Springer. pp. 80–83. ISBN 978-1-4684-4226-7. https://books.google.com/books?id=cW_SBwAAQBAJ&pg=80. Retrieved 17 August 2015. methyldiborane in a gas chromatograph
- Penn, R. E.; Buxton, L. W. (1977). "Microwave spectrum of methyldiborane". The Journal of Chemical Physics 67 (2): 831. doi:10.1063/1.434845. Bibcode: 1977JChPh..67..831P.
- Davidson, G. (1973). "Elements of Group III". Inorganic Chemistry of the Main-Group Elements. Royal Society of Chemistry. p. 57. ISBN 978-0-85186-752-6. https://books.google.com/books?id=5FDADhBgE54C&pg=PA57. Infrared lines
- Isadore Shapiro; C. O. Wilson; J. F. Ditter; W. J. Lehmann (1961). "Mass Spectrometry in Boron Chemistry". Borax to Boranes. Advances in Chemistry Series. 32. American Chemical Society. pp. 135–136. doi:10.1021/ba-1961-0032.ch014. ISBN 978-0-8412-0033-3. https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/4264-BORAX-TO-BORANES3db1.pdf?topic=2297.0. mass spectroscopy
- Kapshtal, V. N.; Sverdlov, L. M. (November 1968). "Sum rules for the frequencies and squares of the frequencies of the vibrations in methyl-substituted diborane derivatives". Soviet Physics Journal 11 (11): 120–122. doi:10.1007/BF00816081. Bibcode: 1968SvPhJ..11k.120K.
 |

