Chemistry:Trimethyldiborane
| |
| Names | |
|---|---|
| IUPAC name
1,1,2-Trimethyldiborane
| |
| Other names
Trimethyldiborane(6)
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| Properties | |
| (CH3)3B2H3 | |
| Molar mass | 69.75 g mol−1 |
| Appearance | Colorless pyrophoric liquid |
| Melting point | −122.9 °C (−189.2 °F; 150.2 K) |
| Boiling point | 45.5 °C (113.9 °F; 318.6 K) |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
48 kcal/mol |
| Related compounds | |
| trimethylborane tetramethyldiborane dimethyldiborane methyldiborane | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
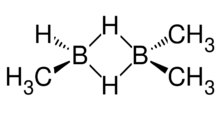
Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane.[1] called 1,2-dimethyldiborane.[2] Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure.[3] The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.[4][5]
Formation
Trimethylborane is formed by the reaction of diborane and trimethylborane. This reaction produces four different substitution of methyl with hydrogen on diborane. Produced is 1-methyldiborane, 1,1-dimethyldborane, 1,1,2-trimethyldiborane and 1,1,2,2-tetramethyldiborane. By reacting monomethyldiborane with ether, dimethylether borine is formed [(CH3)2O].BH3 leaving methylborane which rapidly dimerises to 1,2-dimethyldiborane.[3] The reaction is complex.[6] The yield of trimethyldiborane is maximised with ratio of 1 of diborane to 3 of trimethylborane.[7]
Tetramethyl lead can react with diborane in a 1,2-dimethoxyethane solvent at room temperature to make a range of methyl substituted diboranes, ending up at trimethylborane, but including 1,1-di, tridiborane. The other outputs of the reaction are hydrogen gas and lead metal.[8]
Other methods to form methyldiboranes include reacting hydrogen with trimethylborane between 80 and 200 °C under pressure, or reacting a metal borohydride with trimethylborane in the presence of hydrogen chloride, aluminium chloride or boron trichloride. If the borohydride is sodium borohydride, then methane is a side product. If the metal is lithium then no methane is produced.[4] Dimethylchloroborane and methyldichloroborane are also produced as gaseous products.[4]
When Cp2Zr(CH3)2 reacts with borane dissolved in tetrahydrofuran, a borohydro group inserts into the zirconium carbon bond, and methyl diboranes are produced.[9]
Properties
Trimethyldiborane has two methyl groups on one boron atom, and one methyl and a hydrogen on the second boron atom. A bridge of two hydrogen atoms links the boron atoms together. The molecule is expected to have a Cs point group due to rapid rotation of the methyls. The infrared spectrum of trimethyldiborane has a strong absorption band at 2509 cm−1 due to the non-bridge boron-hydrogen bond.[10] It has a vapour pressure of 51 mm Hg at -22.8 °C; 61 mm Hg at -18.4 °C and[7] 83 mm Hg at 0 °C.[11] Vapour pressure can be approximated by Log P = 7.673 - (1527/T).[12] The boiling point is 45.5 °C, and the melting point is -122.9.[12]
The predicted heat of formation for liquid trimethyldiborane is ΔH0f=-48 kcal/mol, and for the gas -41 kcal/mol. Heat of vapourisation ΔHvap was measured at 7.0 kcal/mol.[13]
A gas chromatograph can be used to determine the amounts of the methyl boranes in a mixture. The order they pass through are diborane, monomethyldiborane, trimethylborane, 1,1-dimethyldiborane, 1,2-dimethyldiborane, trimethyldiborane, and lastly tetramethyldiborane.[14]
The nuclear resonance shift for the bridge hydrogen is 9.27 ppm, compared to 10.49 for diborane.[15]
Reactions
Trimethyldiborane partially disproportionates over a period of hours at room temperature to yield tetramethyldiborane and 1,2-dimethyldiborane. Over a period of weeks 1,1-dimethyldiborane appears as well.[16]
Trimethyldiborane is hydrolyzed in water to methylboronic acid CH3B(OH)2 and dimethylborinic acid (CH3)2B(OH).[3]
Trimethyldiborane spontaneously inflames when exposed to air.[18]
Trimethyldiborane reacts with liquid ammonia initially forming methylborohydride anions and (CH3)2B(N3)2+ cations.[19][20][21]
Related
Trimethylborane (CH3)3B has a similar-sounding name, and many similar properties, but only has one boron atom.[4] Trimethylhydroborate (CH3)3BH− is an anion with one boron atom. It can form a lithium salt.[22]
References
- ↑ Srebnik, Morris; Cole, Thomas E.; Brown, Herbert C. (January 1987). "Methylborane - a remarkable unhindered monoalkylborane which achieves the controlled sequential hydroboration of representative alkenes". Tetrahedron Letters 28 (33): 3771–3774. doi:10.1016/s0040-4039(00)96380-9.
- ↑ Low, M. J. D. (1968). "Characteristic Infrared Frequencies of Methyldiboranes". The Journal of Chemical Physics 48 (5): 2386–2387. doi:10.1063/1.1669454. Bibcode: 1968JChPh..48.2386L.
- ↑ 3.0 3.1 3.2 Bell, R. P.; Emeléus, H. J. (1948). "The boron hydrides and related compounds". Quarterly Reviews, Chemical Society 2 (2): 132. doi:10.1039/QR9480200132.
- ↑ 4.0 4.1 4.2 4.3 Long, L. H.; Wallbridge, M. G. H. (1965). "646. The chemistry of boron. Part VI. New preparative methods and decomposition studies relating to methyldiboranes". Journal of the Chemical Society (Resumed): 3513–3520. doi:10.1039/JR9650003513. http://pubs.rsc.org/en/content/articlepdf/1965/jr/jr9650003513. (Subscription content?)
- ↑ Schlesinger, H. I.; Walker, A. O. (April 1935). "Hydrides of Boron. IV. The Methyl Derivatives of Diborane". Journal of the American Chemical Society 57 (4): 621–625. doi:10.1021/ja01307a009.
- ↑ van Aalten, Lloyd; Seely, G. R.; Oliver, Juhn; Ritter, D. M. (1 June 1961). Kinetics and Equilibria in the Alkylation of Diborane Preliminary Report. Advances in Chemistry. 32. American Chemical Society. pp. 107–114. doi:10.1021/ba-1961-0032.ch012. ISBN 0-8412-0033-5. https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/4264-BORAX-TO-BORANES3db1.pdf?topic=2297.0.
- ↑ 7.0 7.1 Carpenter, J.H.; Jones, W.J.; Jotham, R.W.; Long, L.H. (September 1971). "The Raman spectra of the methyldiboranes—II Monomethyldiborane and trimethyldiborane, and characteristic frequencies of the methyldiboranes". Spectrochimica Acta Part A: Molecular Spectroscopy 27 (9): 1721–1734. doi:10.1016/0584-8539(71)80227-1. Bibcode: 1971AcSpA..27.1721C.
- ↑ Holliday, A.K.; N. Jessop, G. (November 1967). "The reaction of tetramethyllead with diborane". Journal of Organometallic Chemistry 10 (2): 291–293. doi:10.1016/s0022-328x(00)93089-4.
- ↑ Marsella, John A.; Caulton, Kenneth G. (May 1982). "Dealkylation of zirconium(IV) by borane: the intimate mechanism of an alkyl transfer reaction". Journal of the American Chemical Society 104 (9): 2361–2365. doi:10.1021/ja00373a005.
- ↑ Cowan, R. D. (1949). "The Infra-Red Spectra of Borine Carbonyl and Tetramethyldiborane". The Journal of Chemical Physics 17 (2): 218. doi:10.1063/1.1747225. Bibcode: 1949JChPh..17..218C.
- ↑ "Thermal reaction of diborane with trimethylborane". National Advisory Committee for Aeronautics. 4 September 1958. https://digital.library.unt.edu/ark:/67531/metadc64257/m1/1/.
- ↑ 12.0 12.1 Onak, Thomas (1 January 1966). Stone, F. G. A.; West, Robert. eds. Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. ISBN 9780080580043. https://books.google.com/books?id=AHEPoIx22iwC&pg=PA284. Retrieved 14 August 2015.
- ↑ Altschuller, Aubrey P. (4 October 1955). "Calculated Heats of Formation and Combustion of Boron Compounds (Boron, Hydrogen, Carbon, Silicon)". Cleveland, Ohio: National Advisory Committee for Aeronautics. p. 22. http://naca.central.cranfield.ac.uk/reports/1955/naca-rm-e55g26.pdf.
- ↑ Seely, G. R.; Oliver, J. P.; Ritter, D. M. (December 1959). "Gas-Liquid Chromatographic Analysis of Mixtures Containing Methyldiboranes". Analytical Chemistry 31 (12): 1993–1995. doi:10.1021/ac60156a032.
- ↑ Leach, John B.; Ungermann, Charles B.; Onak, Thomas P. (January 1972). "Proton magnetic resonance studies on methyl and chloro substituted diboranes". Journal of Magnetic Resonance 6 (1): 74–83. doi:10.1016/0022-2364(72)90088-1. Bibcode: 1972JMagR...6...74L.
- ↑ Lehmann, Walter J.; Wilson, Charles O.; Shapiro, I. (1961). "Infrared Spectra of Alkyldiboranes. V. Tri- and Tetramethyl- and Ethyldiboranes". The Journal of Chemical Physics 34 (3): 783. doi:10.1063/1.1731675. Bibcode: 1961JChPh..34..783L.
- ↑ 17.0 17.1 Onak, Thomas (1 January 1966). "Carboranes and Organo-Substituted Boron Hydrides". in Stone, F. G. A.; West, Robert. Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. ISBN 9780080580043. https://books.google.com/books?id=AHEPoIx22iwC&pg=PA266. Retrieved 19 August 2015.
- ↑ Urben, Peter; Pitt, M. J., eds (2007). Bretherick's Handbook of Reactive Chemical Hazards (7th ed.). Amsterdam: Elsevier. p. 527. ISBN 978-0-12-373945-2.
- ↑ Jungfleisch, Francis (1973). "Reactions of Methyl Substituted Diboranes and 2,2-Dimethyltetraborane with Amine Bases". Ohio State University. p. 64. https://etd.ohiolink.edu/!etd.send_file?accession=osu1298565601&disposition=inline.
- ↑ Sheldon, J. C.; Smith, B. C. (1960). "The borazoles". Quarterly Reviews, Chemical Society 14 (2): 202. doi:10.1039/QR9601400200.
- ↑ Schlesinger, H. I.; Horvitz, Leo; Burg, A. B. (March 1936). "Hydrides of Boron. VI. The Action of Ammonia on the Methyl Diboranes". Journal of the American Chemical Society 58 (3): 409–414. doi:10.1021/ja01294a008.
- ↑ "inorganic materials research division - The Berkeley Lab". https://publications.lbl.gov/islandora/object/ir%3A103576/datastream/PDF/download/citation.pdf.
Extra reading
- Carpenter, J. H.; Jones, W. J.; Jotham, R. W.; Long, L. H. (1968). "Laser-source Raman spectroscopy and the Raman spectra of the methyldiboranes". Chemical Communications (15): 881. doi:10.1039/C19680000881.
 |

