Chemistry:Neptunocene
| |
| Names | |
|---|---|
| IUPAC name
Bis(η8-cyclooctatetraenyl)neptunium(IV)
| |
| Other names
Neptunium cyclooctatetraenide
Np(COT)2 | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C16H16Np | |
| Molar mass | 445 g·mol−1 |
| Appearance | dark brown crystals as a solid, yellow in dilute solution |
| insoluble, does not react with water | |
| Solubility in chlorocarbons | sparingly soluble (ca. 0.5 g/L) |
| Hazards | |
| Main hazards | radiation hazard, pyrophoric |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
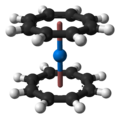
Neptunocene, Np(C8H8)2, is an organoneptunium compound composed of a neptunium atom sandwiched between two cyclooctatetraenide (COT2-) rings. As a solid it has a dark brown/red colour but it appears yellow when dissolved in chlorocarbons, in which it is sparingly soluble.[1][2][3][4][5] The compound is quite air-sensitive.[1][2][5]
It was one of the first organoneptunium compounds to be synthesised, and is a member of the actinocene family of actinide-based metallocenes.[2]
Structure
The sandwich structure of neptunocene has been determined by single crystal XRD.[4] The COT2- rings are found to be planar with 8 equivalent C–C bonds of 1.385 Å length, and sit parallel in an eclipsed conformation. The Np–COT distance (to the ring centroid) is 1.909 Å and the individual Np–C distances are 2.630 Å.[4]
Neptunocene assumes a monoclinic crystal structure (P21/n space group) which is isomorphous to uranocene and thorocene but not to plutonocene.[4]
Synthesis and properties
Neptunocene was first synthesised in 1970 by reacting neptunium(IV) chloride (NpCl4) with dipotassium cyclooctatetraenide (K2(C8H8)) in diethyl ether or THF:[1]
- NpCl4 + 2 K2(C8H8) → Np(C8H8)2 + 4 KCl
The same reaction conditions have been routinely reproduced since then for the synthesis of the compound.[3][4]
The three actinocenes uranocene, neptunocene, and plutonocene share virtually identical chemistry: they do not react in the presence of water or dilute base, but are very air-sensitive, quickly forming oxides.[1][2][3] All three are only slightly soluble (up to about 10−3 M concentrations) in aromatic or chlorinated solvents such as benzene, toluene, carbon tetrachloride or chloroform.[1][2][4][5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Karraker, David G.; Stone, John Austin.; Jones, Erwin Rudolph.; Edelstein, Norman. (1970). "Bis(cyclooctatetraenyl)neptunium(IV) and bis(cyclooctatetraenyl)plutonium(IV)" (in en). Journal of the American Chemical Society 92 (16): 4841–4845. doi:10.1021/ja00719a014. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00719a014.
- ↑ 2.0 2.1 2.2 2.3 2.4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1278–1280. ISBN 9780750633659.
- ↑ 3.0 3.1 3.2 Eisenberg, David C.; Streitwieser, Andrew; Kot, Wing K. (1990). "Electron transfer in organouranium and transuranium systems". Inorganic Chemistry 29 (1): 10–14. doi:10.1021/ic00326a004. ISSN 0020-1669. https://pubs.acs.org/doi/pdf/10.1021/ic00326a004.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Ridder, D. J. A. De; Rebizant, J.; Apostolidis, C.; Kanellakopulos, B.; Dornberger, E. (1996). "Bis(cyclooctatetraenyl)neptunium(IV)" (in en). Acta Crystallographica Section C 52 (3): 597–600. doi:10.1107/S0108270195013047. ISSN 1600-5759. https://onlinelibrary.wiley.com/doi/abs/10.1107/S0108270195013047.
- ↑ 5.0 5.1 5.2 Yoshida, Zenko; Johnson, Stephen G.; Kimura, Takaumi; Krsul, John R. (2006). "Neptunium". in Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements. 3 (3rd ed.). Dordrecht, the Netherlands: Springer. pp. 699–812. doi:10.1007/1-4020-3598-5_6. http://radchem.nevada.edu/classes/rdch710/files/neptunium.pdf. Retrieved 2016-08-07.
 |
