Chemistry:Neptunium(IV) fluoride
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Neptunium(IV) fluoride
| |
| Other names
Neptunium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| NpF4 | |
| Molar mass | 313 g/mol |
| Appearance | Green solid[1] |
| Structure | |
| Monoclinic, mS60[1] | |
| C2/c, No. 15[2] | |
a = 1.27 nm, b = 1.0082 nm, c = 0.833 nm α = 90°, β = 126.03°, γ = 90°
| |
Lattice volume (V)
|
0.86256 nm3 |
Formula units (Z)
|
12 |
| Thermochemistry | |
Heat capacity (C)
|
116 ± 4 J/mol·K[1] |
Std molar
entropy (S |
148 ± 3 J/mol·K[1] |
Std enthalpy of
formation (ΔfH⦵298) |
−1874 ± 16 kJ/mol[1] |
Gibbs free energy (ΔfG˚)
|
-1783 ± 16 kJ/mol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
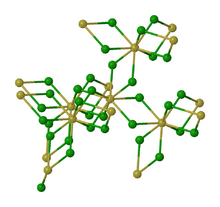
Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.[3]
Synthesis
thumb|left|162px|Alternative view of the structure of solid NpF4. Neptunium(IV) fluoride can be prepared by reacting neptunium(III) fluoride or neptunium dioxide with a gas mixture of oxygen and hydrogen fluoride at 500 °C:[1]
It can also be prepared by treating neptunium dioxide with HF gas:[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Haire, Richard G. (2006). "Neptunium". in Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. pp. 730–736. doi:10.1007/1-4020-3598-5_9. ISBN 1-4020-3555-1.
- ↑ Zachariasen, W. H. (1949). "Crystal chemical studies of the 5f-series of elements. XII. New compounds representing known structure types". Acta Crystallographica 2 (6): 388–390. doi:10.1107/S0365110X49001016.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
 |

