Chemistry:Nickel(II) laurate
From HandWiki
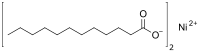
| |
| Names | |
|---|---|
| Other names
nickel(2+) dodecanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C24H46NiO4 | |
| Molar mass | 457.321 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Nickel(II) laurate is an metal-organic compound with the chemical formula C24H46NiO4.[1][2] It is classified as a metallic soap, i.e. a metal derivative of a fatty acid (lauric acid).[3]
Preparation
Reaction of acqueos solutions of nickel salt and soluble laurate.[4] Nickel(II) laurate forms green precipitate.
References
- ↑ "Nickel(II) laurate". National Institute of Advanced Industrial Science and Technology. https://sdbs.db.aist.go.jp/sdbs/cgi-bin/landingpage?sdbsno=16244.
- ↑ Buehler, Calvin Adam; Pearson, Donald Emanual; Pearson, Donald E. (1970) (in en). Survey of Organic Syntheses. Wiley-Interscience. p. 429. ISBN 978-0-471-11671-4. https://books.google.com/books?id=MAYpAQAAMAAJ&q=Nickel(II)+laurate. Retrieved 26 January 2023.
- ↑ Mehrotra, K. N.; Kachhwaha, R. (1 December 1980). "Studies of infrared, diffuse reflectance spectra, colorimetry and thermal analysis of nickel laurate". Tenside Surfactants Detergents 17 (6): 304–306. doi:10.1515/tsd-1980-170613.
- ↑ "Method of preparing nickel laurate" (in en). 19 April 2017. https://patents.google.com/patent/CN106565462A/en.
 |

