Chemistry:Nitrapyrin
| |
| Names | |
|---|---|
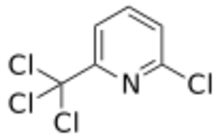
| Preferred IUPAC name
2-Chloro-6-(trichloromethyl)pyridine | |
| Other names
N-serve, 2,2,2,6-Tetrachloro-2-picoline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H3Cl4N | |
| Molar mass | 230.907 |
| Appearance | colorless/white crystalline solid[1] |
| Odor | Sweet[1] |
| Melting point | 63 °C; 145 °F; 336 K[1] |
| insoluble[1] | |
| Vapor pressure | 0.003 mmHg (22.8°C) |
| Hazards | |
| Main hazards | explosive[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
REL (Recommended)
|
TWA 10 mg/m3 (total) ST 20 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitrapyrin is an organic compound with the formula ClC5H3NCCl3, and is described as a white crystalline solid with a sweet odor.[2] It is used as a nitrification inhibitor and bactericide, which is applied to soils for the growing of agricultural crops[3] since 1974. Nitrapyrin was put up for review by the EPA and deemed safe for use in 2005.[4] Nitrapyrin is an effective nitrification inhibitor to the bacteria Nitrosomonas and has been shown to drastically the reduce the amount of N2O emissions from the soil.[3]
Synthesis
Nitrapyrin is commonly produced by the photochlorination of 2-methylpyridine:[5]
- CH3-C5H4N + 4Cl2 → CCl3-ClC5H3N + 4 HCl
Function
Nitrapyrin affects the ammonia monooxygenase (AMO) pathway,[6] which is important for NH3 oxidation in nitrification;[7] it also functions as an inhibitor of the urease enzyme in the nitrifying bacteria Nitrosomonas,[8] preventing hydrolytic action on urea.[9][10] It is applied to the region of soil and inhibits nitrification for 8–10 weeks. Urease Inhibition specifically prevents the following reaction:
(NH2)2CO + H2O → CO2 + 2NH3
Without this capability Nitrosomonas cannot produce nitrite thus inhibiting nitrification:
2NH4+ + 3O2 → 2NO2− + 2 H2O + 4H+
Degradation/Decomposition
Nitrapyrin decomposes both in soil and in plants. The compound itself tends not to persist in nature. The primary decomposition is the hydrolysis of the trichloromethyl functional group, resulting primarily in 6-chloro-picolinic acid[10][11] which is the only detected residue in plant metabolisms.
Effects in Agriculture
In an agricultural setting, nitrapyrin is seen to increase nitrogen retention and decrease nitrogen leaching in root zone.[12] Nitrapyrin also has the effect of increasing crop yield and decreasing emissions of N2O gas.[12][13] Nitrapyrin isn't the only product applied to soils for the growing of crops, when combined with urea and mulch, wheat biomass increased by 33% and overall yield increased by 23%.[13] Total N2O emissions reduced by 66-75% when compared to urea only experiments, suggesting that nitrapyrin affects the ability of ammonia-oxidizing bacteria to engage in nitrification and produce N2O gas.[13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 NIOSH Pocket Guide to Chemical Hazards. "#0136". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0136.html.
- ↑ "2-CHLORO-6-(TRICHLOROMETHYL) PYRIDINE (NITRAPYRIN) | Occupational Safety and Health Administration". https://www.osha.gov/chemicaldata/303.
- ↑ 3.0 3.1 Kiiski, Harri; Dittmar, Heinrich; Drach, Manfred; Vosskamp, Ralf; Trenkel, Martin E.; Gutser, Reinhold; Steffens, Günter (2016-03-21), Wiley-VCH Verlag GmbH & Co. KGaA, ed. (in en), Fertilizers, 2. Types, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. 1–53, doi:10.1002/14356007.n10_n01.pub2, ISBN 978-3-527-30673-2, https://onlinelibrary.wiley.com/doi/10.1002/14356007.n10_n01.pub2, retrieved 2022-04-24
- ↑ "Red facts - Nitrapyrin - US EPA". May 2005. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-069203_1-May-05.pdf.
- ↑ Powell, Steven J.; Prosser, James I. (1986). "Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture". Applied and Environmental Microbiology 52 (4): 782–787. doi:10.1128/aem.52.4.782-787.1986. PMID 16347171. Bibcode: 1986ApEnM..52..782P.
- ↑ Subbarao, G. V.; Nakahara, K.; Hurtado, M. P.; Ono, H.; Moreta, D. E.; Salcedo, A. F.; Yoshihashi, A. T.; Ishikawa, T. et al. (October 13, 2009). "Evidence for biological nitrification inhibition in Brachiaria pastures". Proceedings of the National Academy of Sciences 106 (41): 17302–17307. doi:10.1073/pnas.0903694106. ISSN 0027-8424. PMID 19805171. Bibcode: 2009PNAS..10617302S.
- ↑ Zhou, Xue; Wang, Shuwei; Ma, Shutan; Zheng, Xinkun; Wang, Zhiyuan; Lu, Chunhui (December 2020). "Effects of commonly used nitrification inhibitors—dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP), and nitrapyrin—on soil nitrogen dynamics and nitrifiers in three typical paddy soils". Geoderma 380: 114637. doi:10.1016/j.geoderma.2020.114637. ISSN 0016-7061. Bibcode: 2020Geode.380k4637Z. http://dx.doi.org/10.1016/j.geoderma.2020.114637.
- ↑ Z. Amtul, Atta-ur-Rahman, R. A. Siddiqui, M. I. Choudhary; "Chemistry and Mechanism of Urease Inhibition" Curr Med Chem. 2002 Jul;9(14):1323-48.
- ↑ M.E. Trenkel Slow- and Controlled-Release and Stabilizing Fertilizers An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; IFA: Paris, 2010 ISBN:978-2-9523139-7-1
- ↑ 10.0 10.1 JHG Slangen, P. Kerkhoff; Nitrification Inhibitors in Agriculture and Horticulture: A Literature Review
- ↑ John H. Montgomery Agrochemicals Desk Reference 2nd ed.; Boca Raton: CRC Press, 1997 ISBN:1-56670-167-8
- ↑ 12.0 12.1 Wolt, Jeffrey D. (May 2004). "A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the Midwestern USA" (in en). Nutrient Cycling in Agroecosystems 69 (1): 23–41. doi:10.1023/B:FRES.0000025287.52565.99. ISSN 1385-1314. http://link.springer.com/10.1023/B:FRES.0000025287.52565.99.
- ↑ 13.0 13.1 13.2 Dawar, Khadim; Khan, Aamir; Sardar, Kamil; Fahad, Shah; Saud, Shah; Datta, Rahul; Danish, Subhan (February 2021). "Effects of the nitrification inhibitor nitrapyrin and mulch on N2O emission and fertilizer use efficiency using 15N tracing techniques". Science of the Total Environment 757: 143739. doi:10.1016/j.scitotenv.2020.143739. ISSN 0048-9697. PMID 33229088. Bibcode: 2021ScTEn.757n3739D. http://dx.doi.org/10.1016/j.scitotenv.2020.143739.
 |
