Chemistry:Nitroacetanilide
From HandWiki
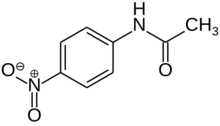
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(4-Nitrophenyl)acetamide | |
| Other names
p-Acetamidonitrobenzene; p-Nitroacetanilide; N-Acetyl-4-nitroaniline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H8N2O3 | |
| Molar mass | 180.16 g/mol |
| Appearance | Solid, white-green or brown |
| Density | 1.34 g/cm3 |
| Melting point | 215 °C (419 °F; 488 K) |
| Boiling point | 408.9 °C (768.0 °F; 682.0 K) |
| Hazards[1] | |
| Main hazards | Irritant |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Nitroacetanilide is a chemical compound which is a nitro derivative of acetanilide. There are two other isomers of nitroacetanilide, 2-nitroacetanilide and 3-nitroacetanilide.
4-Nitroacetanilide is used as in intermediate in the production of some dyes.[2]
References
- ↑ "N-(4-Nitrophenyl)acetamide (compound)". https://pubchem.ncbi.nlm.nih.gov/compound/7691#section=Safety-and-Hazards.
- ↑ "Dyes and Dye Intermediates" in Kirk‑Othmer Encyclopedia of Chemical Technology, Peter Gregory, doi:10.1002/0471238961.0425051907180507.a01
 |


