Chemistry:Nitronium tetrafluoroborate
From HandWiki
| |
| |
| Names | |
|---|---|
| Other names
nitronium fluoroborate, NO2BF4
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| BNO2F4 | |
| Molar mass | 132.81 |
| Hazards | |
| Safety data sheet | [1] |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H314, H317, H334 | |
| P260, P261, P264, P272, P280, P285, P301+330+331, P302+352, P303+361+353, P304+340, P304+341, P305+351+338, P310, P321, P333+313, P342+311, P363, P405, P501 | |
| Related compounds | |
Other cations
|
Nitrosonium tetrafluoroborate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
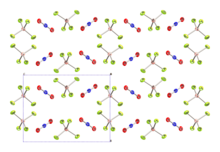
Nitronium tetrafluoroborate is an inorganic compound with formula NO2BF4. It is a salt of nitronium cation and tetrafluoroborate anion. It is a colorless crystalline solid, which reacts with water to form the corrosive acids HF and HNO3. As such, it must be handled under water-free conditions. It is sparsely soluble in many organic solvents.
Preparation
Nitronium tetrafluoroborate can be prepared by adding a mixture of anhydrous hydrogen fluoride and boron trifluoride to a nitromethane solution of nitric acid or dinitrogen pentoxide.[1]
Applications
Nitronium tetrafluoroborate is used as a nitration agent.
References
- ↑ Kenneth Schofield (1980). Aromatic nitration. CUP Archive. p. 88. ISBN 0-521-23362-3.
 |

