Chemistry:Nitryl cyanide
From HandWiki
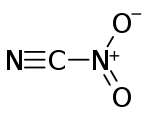
| |
| Names | |
|---|---|
| IUPAC name
nitroformonitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| CN2O2 | |
| Molar mass | 72.023 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.24 g ml−1 (−79 °C) |
| Melting point | −85 °C (−121 °F; 188 K) |
| Boiling point | 7 °C (45 °F; 280 K) |
| Reacts with water | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
212 kJ mol−1 |
| Related compounds | |
Related compounds
|
Nitrile isocyanide (CNNO2) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Nitryl cyanide is an energetic chemical compound with the formula NCNO2. Nitryl cyanide is a possible precursor to the theoretical explosive 2,4,6-trinitro-1,3,5-triazine.[1][2]
Synthesis
Nitryl cyanide was first synthesized in 2014. The reaction of nitronium tetrafluoroborate with tert-butyldimethylsilyl cyanide at −30 °C produces nitryl cyanide, with tert-butyldimethylsilyl fluoride and boron trifluoride as byproducts.[1]
- NO
2BF
4 + t–BuMe
2SiCN → NCNO
2 + t–BuMe
2SiF + BF
3
The conversion of this method is only 50%, and using an excess of tert-butyldimethylsilyl causes the yield to drop even further.[1]
References
- ↑ 1.0 1.1 1.2 Rahm, Martin; Bélanger-Chabot, Guillaume; Haiges, Ralf; Christe, Karl O. (2014-07-01). "Nitryl Cyanide, NCNO2" (in en). Angewandte Chemie International Edition 53 (27): 6893–6897. doi:10.1002/anie.201404209. PMID 24861214. https://onlinelibrary.wiley.com/doi/10.1002/anie.201404209.
- ↑ Zhou, Ming-Ming; Xiang, Dong (2022-05-29). "Theoretical Prediction of Structures and Properties of 2,4,6-Trinitro-1,3,5-Triazine (TNTA) Green Energetic Materials from DFT and ReaxFF Molecular Modeling" (in en). Materials 15 (11): 3873. doi:10.3390/ma15113873. ISSN 1996-1944. PMID 35683171. Bibcode: 2022Mate...15.3873Z.
 |

