Chemistry:Octopine
![Stereo, skeletal formula of octopine ((2S)-2-{[(1R)-1-carboxyethyl]amino})](/wiki/images/thumb/4/43/Octopine_structure.svg/220px-Octopine_structure.svg.png)
| |
| Names | |
|---|---|
| IUPAC name
2-[[1-Carboxyethyl]amino]-5-(diaminomethylideneamino)pentanoic acid[citation needed]
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | octopine |
PubChem CID
|
|
| |
| |
| Properties | |
| C9H18N4O4 | |
| Molar mass | 246.267 g·mol−1 |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octopine is a derivative of the amino acids arginine and alanine. It was the first member of the class of chemical compounds known as opines to be discovered. Octopine gets its name from Octopus octopodia from which it was first isolated in 1927.[1]
Octopine has been isolated from the muscle tissue of invertebrates such as octopus, Pecten maximus and Sipunculus nudus where it functions as an analog of lactic acid.[2] Plants may also produce this compound after infection by Agrobacterium tumefaciens and transfer of the octopine synthesis gene from the bacterium to the plant. [3]
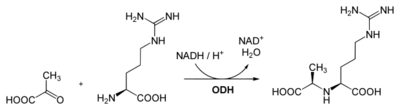
Octopine is formed by reductive condensation of pyruvic acid and arginine through the action of the NADH-dependent enzyme octopine dehydrogenase (ODH).[4] The reaction is reversible so that pyruvic acid and arginine can be regenerated.
References
- ↑ Morizawa, Kiyoshi (1927). "The extractive substances in Octopus octopodia". Acta Scholae Medicinalis Universitatis Imperialis in Kioto 9: 285–298.
- ↑ Hockachka, P.; Hartline, P.; Fields, J. (1977). "Octopine as an end product of anaerobic glycolysis in the chambered nautilus". Science 195 (4273): 72–4. doi:10.1126/science.831256. PMID 831256. Bibcode: 1977Sci...195...72H.
- ↑ Hooykaas, Paul J. J.; Schilperoort, Rob A. (1 May 1992). "Agrobacterium and plant genetic engineering". Plant Molecular Biology 19 (1): 15–38. doi:10.1007/BF00015604. PMID 1600167.
- ↑ Smits, Sander H.J.; Mueller, Andre; Schmitt, Lutz; Grieshaber, Manfred K. (2008). "A Structural Basis for Substrate Selectivity and Stereoselectivity in Octopine Dehydrogenase from Pecten maximus". Journal of Molecular Biology 381 (1): 200–11. doi:10.1016/j.jmb.2008.06.003. PMID 18599075.
 |