Biology:Agrobacterium
| Agrobacterium | |
|---|---|
| |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Alphaproteobacteria |
| Order: | Hyphomicrobiales |
| Family: | Rhizobiaceae |
| Genus: | Agrobacterium Conn 1942 (Approved Lists 1980) |
| Type species | |
| Agrobacterium radiobacter (Smith and Townsend 1907) Conn 1942 (Approved Lists 1980)
| |
| Species | |
| |
| Synonyms[1] | |
| |
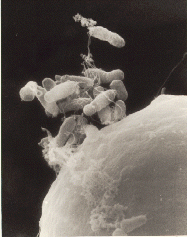
Agrobacterium is a genus of Gram-negative bacteria established by H. J. Conn that uses horizontal gene transfer to cause tumors in plants. Agrobacterium tumefaciens is the most commonly studied species in this genus. Agrobacterium is well known for its ability to transfer DNA between itself and plants, and for this reason it has become an important tool for genetic engineering.
Nomenclatural History
Leading up to the 1990s, the genus Agrobacterium was used as a wastebasket taxon. With the advent of 16S sequencing, many Agrobacterium species (especially the marine species) were reassigned to genera such as Ahrensia, Pseudorhodobacter, Ruegeria, and Stappia.[2][3] The remaining Agrobacterium species were assigned to three biovars: biovar 1 (Agrobacterium tumefaciens), biovar 2 (Agrobacterium rhizogenes), and biovar 3 (Agrobacterium vitis). In the early 2000s, Agrobacterium was synonymized with the genus Rhizobium.[4] This move proved to be controversial.[5][6] The debate was finally resolved when the genus Agrobacterium was reinstated[7] after it was demonstrated that it was phylogenetically distinct from Rhizobium[8][9] and that Agrobacterium species were unified by a unique synapomorphy: the presence of the protelomerase gene, telA, which causes all members of the genus to have a linear chromid.[10] By this time, however, the three Agrobacterium biovars had become defunct; biovar 1 remained with Agrobacterium, biovar 2 was renamed Rhizobium rhizogenes, and biovar 3 was renamed Allorhizobium vitis.
Plant pathogen

Agrobacterium tumefaciens causes crown-gall disease in plants. The disease is characterised by a tumour-like growth or gall on the infected plant, often at the junction between the root and the shoot. Tumors are incited by the conjugative transfer of a DNA segment (T-DNA) from the bacterial tumour-inducing (Ti) plasmid. The closely related species, Agrobacterium rhizogenes, induces root tumors, and carries the distinct Ri (root-inducing) plasmid. Although the taxonomy of Agrobacterium is currently under revision it can be generalised that 3 biovars exist within the genus, Agrobacterium tumefaciens, Agrobacterium rhizogenes, and Agrobacterium vitis. Strains within Agrobacterium tumefaciens and Agrobacterium rhizogenes are known to be able to harbour either a Ti or Ri-plasmid, whilst strains of Agrobacterium vitis, generally restricted to grapevines, can harbour a Ti-plasmid. Non-Agrobacterium strains have been isolated from environmental samples which harbour a Ri-plasmid whilst laboratory studies have shown that non-Agrobacterium strains can also harbour a Ti-plasmid. Some environmental strains of Agrobacterium possess neither a Ti nor Ri-plasmid. These strains are avirulent.[11]
The plasmid T-DNA is integrated semi-randomly into the genome of the host cell,[12] and the tumor morphology genes on the T-DNA are expressed, causing the formation of a gall. The T-DNA carries genes for the biosynthetic enzymes for the production of unusual amino acids, typically octopine or nopaline. It also carries genes for the biosynthesis of the plant hormones, auxin and cytokinins, and for the biosynthesis of opines, providing a carbon and nitrogen source for the bacteria that most other micro-organisms can't use, giving Agrobacterium a selective advantage.[13] By altering the hormone balance in the plant cell, the division of those cells cannot be controlled by the plant, and tumors form. The ratio of auxin to cytokinin produced by the tumor genes determines the morphology of the tumor (root-like, disorganized or shoot-like).
In humans
Although generally seen as an infection in plants, Agrobacterium can be responsible for opportunistic infections in humans with weakened immune systems,[14][15] but has not been shown to be a primary pathogen in otherwise healthy individuals. One of the earliest associations of human disease caused by Agrobacterium radiobacter was reported by Dr. J. R. Cain in Scotland (1988).[16] A later study suggested that Agrobacterium attaches to and genetically transforms several types of human cells by integrating its T-DNA into the human cell genome. The study was conducted using cultured human tissue and did not draw any conclusions regarding related biological activity in nature.[17]
Uses in biotechnology
The ability of Agrobacterium to transfer genes to plants and fungi is used in biotechnology, in particular, genetic engineering for plant improvement. Genomes of plants and fungi can be engineered by use of Agrobacterium for the delivery of sequences hosted in T-DNA binary vectors. A modified Ti or Ri plasmid can be used. The plasmid is 'disarmed' by deletion of the tumor inducing genes; the only essential parts of the T-DNA are its two small (25 base pair) border repeats, at least one of which is needed for plant transformation.[18][19] The genes to be introduced into the plant are cloned into a plant binary vector that contains the T-DNA region of the disarmed plasmid, together with a selectable marker (such as antibiotic resistance) to enable selection for plants that have been successfully transformed. Plants are grown on media containing antibiotic following transformation, and those that do not have the T-DNA integrated into their genome will die. An alternative method is agroinfiltration.[20][21]

Transformation with Agrobacterium can be achieved in multiple ways. Protoplasts or alternatively leaf-discs can be incubated with the Agrobacterium and whole plants regenerated using plant tissue culture. In agroinfiltration the Agrobacterium may be injected directly into the leaf tissue of a plant. This method transforms only cells in immediate contact with the bacteria, and results in transient expression of plasmid DNA.[22]
Agroinfiltration is commonly used to transform tobacco (Nicotiana). A common transformation protocol for Arabidopsis is the floral dip method:[23] inflorescence are dipped in a suspension of Agrobacterium, and the bacterium transforms the germline cells that make the female gametes. The seeds can then be screened for antibiotic resistance (or another marker of interest), and plants that have not integrated the plasmid DNA will die when exposed to the correct condition of antibiotic.[20]
Agrobacterium does not infect all plant species, but there are several other effective techniques for plant transformation including the gene gun.
Agrobacterium is listed as being the vector of genetic material that was transferred to these USA GMOs:[24]
- Soybean
- Cotton
- Maize
- Sugar Beet
- Alfalfa
- Wheat
- Rapeseed Oil (Canola)
- Creeping bentgrass (for animal feed)
- Rice (Golden Rice)
The transformation of fungi using Agrobacterium is used primarily for research purposes,[25][26] and follows similar approaches as for plant transformation. The Ti plasmid system is modified to include DNA elements to select for transformed fungal strains, after co-incubation of Agrobacterium strains carrying these plasmids with fungal species.
Genomics
The sequencing of the genomes of several species of Agrobacterium has permitted the study of the evolutionary history of these organisms and has provided information on the genes and systems involved in pathogenesis, biological control and symbiosis. One important finding is the possibility that chromosomes are evolving from plasmids in many of these bacteria. Another discovery is that the diverse chromosomal structures in this group appear to be capable of supporting both symbiotic and pathogenic lifestyles. The availability of the genome sequences of Agrobacterium species will continue to increase, resulting in substantial insights into the function and evolutionary history of this group of plant-associated microbes.[27]
History
Marc Van Montagu and Jozef Schell at the University of Ghent (Belgium) discovered the gene transfer mechanism between Agrobacterium and plants, which resulted in the development of methods to alter Agrobacterium into an efficient delivery system for gene engineering in plants.[18][19] A team of researchers led by Mary-Dell Chilton were the first to demonstrate that the virulence genes could be removed without adversely affecting the ability of Agrobacterium to insert its own DNA into the plant genome (1983).[28]
See also
- Agroinfiltration
- Marc Van Montagu
- Rhizobium rhizogenes (formerly Agrobacterium rhizogenes)
References
- ↑ Buchanan RE (1965). "Proposal for rejection of the generic name Polymonas Lieske 1928". International Bulletin of Bacteriological Nomenclature and Taxonomy 15 (1): 43–44. doi:10.1099/00207713-15-1-43.
- ↑ "Phylogenetic position of the marine subdivision of Agrobacterium species based on 16S rRNA sequence analysis". The Journal of General and Applied Microbiology 43 (4): 243–247. August 1997. doi:10.2323/jgam.43.243. PMID 12501326.
- ↑ "Reclassification of marine Agrobacterium species: Proposals of Stappia stellulata gen. nov., comb. nov., Stappia aggregata sp. nov., nom. rev., Ruegeria atlantica gen. nov., comb. nov., Ruegeria gelatinovora comb. nov., Ruegeria algicola comb. nov., and Ahrensia kieliense gen. nov., sp. nov., nom. rev.". The Journal of General and Applied Microbiology 44 (3): 201–210. June 1998. doi:10.2323/jgam.44.201. PMID 12501429.
- ↑ "A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis". International Journal of Systematic and Evolutionary Microbiology 51 (Pt 1): 89–103. January 2001. doi:10.1099/00207713-51-1-89. PMID 11211278.
- ↑ "Agrobacterium is a definable genus of the family Rhizobiaceae". International Journal of Systematic and Evolutionary Microbiology 53 (Pt 5): 1681–1687. September 2003. doi:10.1099/ijs.0.02445-0. PMID 13130068.
- ↑ "Classification and nomenclature of Agrobacterium and Rhizobium". International Journal of Systematic and Evolutionary Microbiology 53 (Pt 5): 1689–1695. September 2003. doi:10.1099/ijs.0.02762-0. PMID 13130069.
- ↑ "History and current taxonomic status of genus Agrobacterium". Syst Appl Microbiol 43 (1): 126046. 2020. doi:10.1016/j.syapm.2019.126046. PMID 31818496.
- ↑ "Phylogeny of the Rhizobium-Allorhizobium-Agrobacterium clade supports the delineation of Neorhizobium gen. nov.". Syst Appl Microbiol 37 (3): 208–215. 2014. doi:10.1016/j.syapm.2013.12.007. PMID 24581678.
- ↑ "Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations". Syst Appl Microbiol 38 (2): 84–90. 2015. doi:10.1016/j.syapm.2014.12.003. PMID 25595870.
- ↑ "Single acquisition of protelomerase gave rise to speciation of a large and diverse clade within the Agrobacterium/Rhizobium supercluster characterized by the presence of a linear chromid". Mol Phylogenet Evol 73: 202–207. 2014. doi:10.1016/j.ympev.2014.01.005. PMID 24440816.
- ↑ "Proposal for rejection of Agrobacterium tumefaciens and revised descriptions for the genus Agrobacterium and for Agrobacterium radiobacter and Agrobacterium rhizogenes". International Journal of Systematic Bacteriology 43 (4): 694–702. October 1993. doi:10.1099/00207713-43-4-694. PMID 8240952.
- ↑ "Identification of Arabidopsis thaliana transformants without selection reveals a high occurrence of silenced T-DNA integrations". The Plant Journal 41 (3): 464–77. February 2005. doi:10.1111/j.1365-313X.2004.02312.x. PMID 15659104.
- ↑ "New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation". The EMBO Journal 29 (6): 1021–32. March 2010. doi:10.1038/emboj.2010.8. PMID 20150897.
- ↑ "Agrobacterium infections in humans: experience at one hospital and review". Clinical Infectious Diseases 16 (1): 112–7. January 1993. doi:10.1093/clinids/16.1.112. PMID 8448285.
- ↑ "Recovery of a strain of Agrobacterium radiobacter with a mucoid phenotype from an immunocompromised child with bacteremia". Journal of Clinical Microbiology 31 (9): 2541–3. September 1993. doi:10.1128/JCM.31.9.2541-2543.1993. PMID 8408587.
- ↑ "A case of septicaemia caused by Agrobacterium radiobacter". The Journal of Infection 16 (2): 205–6. March 1988. doi:10.1016/s0163-4453(88)94272-7. PMID 3351321.
- ↑ "Genetic transformation of HeLa cells by Agrobacterium". Proceedings of the National Academy of Sciences of the United States of America 98 (4): 1871–6. February 2001. doi:10.1073/pnas.041327598. PMID 11172043. Bibcode: 2001PNAS...98.1871K.
- ↑ 18.0 18.1 Schell, J.; Van Montagu, M. (1977). "The Ti-Plasmid of Agrobacterium Tumefaciens, A Natural Vector for the Introduction of NIF Genes in Plants?". in Hollaender, Alexander; Burris, R. H.; Day, P. R. et al.. Genetic Engineering for Nitrogen Fixation. Basic Life Sciences. 9. pp. 159–79. doi:10.1007/978-1-4684-0880-5_12. ISBN 978-1-4684-0882-9.
- ↑ 19.0 19.1 "Genetic analysis of transfer and stabilization of Agrobacterium DNA in plant cells". The EMBO Journal 2 (12): 2151–60. 1983. doi:10.1002/j.1460-2075.1983.tb01716.x. PMID 16453483.
- ↑ 20.0 20.1 Thomson JA. "Genetic Engineering of Plants". Biotechnology 3. http://www.eolss.net/sample-chapters/c17/e6-58-03-04.pdf. Retrieved 17 July 2016.
- ↑ "Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins". Journal of Visualized Experiments 77 (77). July 2013. doi:10.3791/50521. PMID 23913006.
- ↑ "Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana". Journal of Visualized Experiments (86). April 2014. doi:10.3791/51204. PMID 24796351.
- ↑ "Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana". The Plant Journal 16 (6): 735–43. December 1998. doi:10.1046/j.1365-313x.1998.00343.x. PMID 10069079.
- ↑ The FDA List of Completed Consultations on Bioengineered Foods
- ↑ "Agrobacterium-mediated transformation as a tool for functional genomics in fungi". Current Genetics 48 (1): 1–17. July 2005. doi:10.1007/s00294-005-0578-0. PMID 15889258.
- ↑ "Agrobacterium-mediated transformation of fungi". Fungal Biology and Biotechnology 4: 6. 2017. doi:10.1186/s40694-017-0035-0. PMID 28955474.
- ↑ Setubal, Joao C.; Wood, Derek; Burr, Thomas; Farrand, Stephen K.; Goldman, Barry S.; Goodner, Brad; Otten, Leon; Slater, Steven (2009). "The Genomics of Agrobacterium: Insights into its Pathogenicity, Biocontrol, and Evolution". in Jackson, Robert W.. Plant Pathogenic Bacteria: Genomics and Molecular Biology. Caister Academic Press. pp. 91–112. ISBN 978-1-904455-37-0. https://books.google.com/books?id=3nySn5qljjMC&pg=PA91.
- ↑ Chilton, Mary-Dell (2001). "Agrobacterium. A Memoir". Plant Physiology 125 (1): 9–14. doi:10.1104/pp.125.1.9. ISSN 0032-0889. PMID 11154285. PMC 1539314. https://www.jstor.org/stable/4279598.
Further reading
- "The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: An example of a naturally transgenic food crop". Proceedings of the National Academy of Sciences of the United States of America 112 (18): 5844–9. May 2015. doi:10.1073/pnas.1419685112. PMID 25902487. Bibcode: 2015PNAS..112.5844K.
- Lay summary in: Bob Yirka (April 21, 2015). "Researchers find the genome of the cultivated sweet potato has bacterial DNA". http://phys.org/news/2015-04-genome-cultivated-sweet-potato-bacterial.html.
External links
- Current taxonomy of Agrobacterium species, and new Rhizobium names
- Agrobacteria is used as gene ferry - Plant transformation with Agrobacterium]
Wikidata ☰ Q2700446 entry
pl:Agrobacterium tumefaciens
 |
