Chemistry:P-Toluic acid
From HandWiki
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Methylbenzoic acid | |||
| Other names
para-toluic acid
p-toluic acid para-methylbenzoic acid p-methylbenzoic acid toluene-4-carboxylic acid crithminic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3904552 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C8H8O2 | |||
| Molar mass | 136.150 g·mol−1 | ||
| Appearance | Crystalline solid | ||
| Density | 1.06g/mL | ||
| Melting point | 180 to 181 °C (356 to 358 °F; 453 to 454 K)[1] | ||
| Boiling point | 274 to 275 °C (525 to 527 °F; 547 to 548 K)[1] | ||
| Sparingly soluble in hot water | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-429 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
3862 kJ/mol | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H302, H315, H317, H319, H335 | |||
| P261, P264, P270, P271, P272, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
p-Toluic acid (4-methylbenzoic acid) is a substituted benzoic acid with the formula CH3C6H4CO2H. It is a white solid that is poorly soluble in water but soluble in acetone. A laboratory route to p-toluic acid involves oxidation of p-cymene with nitric acid.[2]
Role in production of terephthalic acid
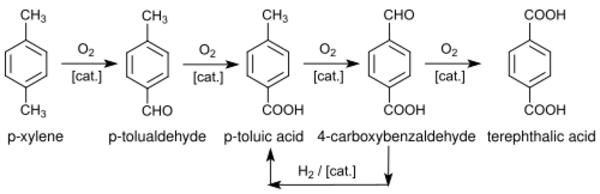
p-Toluic acid is an intermediate in the conversion of p-xylene to terephthalic acid, a commodity chemical used in the manufacture of polyethylene terephthalate. It is generated both by the oxidation of p-xylene as well as the hydrogenolysis of 4-carboxybenzaldehyde. In related processes it is converted to methyl p-toluate, which is oxidized to monomethyl terephthalate.[3]
See also
References
- ↑ 1.0 1.1 Merck Index, 12th Edition, 9673
- ↑ W. F. Tuley; C. S. Marvel (1947). "p-Toluic Acid". Org. Synth. 27: 86. doi:10.15227/orgsyn.027.0086.
- ↑ Tomas, Rogerio A. F.; Bordado, Joao C. M.; Gomes, Joao F. P. (2013). "p-Xylene Oxidation to Terephthalic Acid: A Literature Review Oriented toward Process Optimization and Development". Chemical Reviews 113 (10): 7421–7469. doi:10.1021/cr300298j. PMID 23767849.
External links
 |