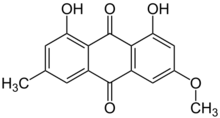
Chemistry:Parietin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,8-Dihydroxy-3-methoxy-6-methylanthracene-9,10 dione | |
| Other names
Physcion(e), rheochrysidin, methoxyemodin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H12O5 | |
| Molar mass | 284.26348 g/mol |
| Appearance | Orange/yellow |
| Related compounds | |
Related compounds
|
Emodin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Parietin is the predominant cortical pigment of lichens in the genus Caloplaca, a secondary product of the lichen Xanthoria parietina, and a pigment found in the roots of curled dock (Rumex crispus). It has an orange-yellow color and absorbs blue light.
It is also known as physcion.
It has also been shown to protect lichens against UV-B light, at high altitudes in alpine regions. The UV-B light stimulates production of parietin and the parietin protects the lichens from damage. Lichens in arctic regions such as Svalbard retain this capability though they do not encounter damaging levels of UV-B, a capability that could help protect the lichens in case of ozone layer thinning.[1][2][3]
It has also shown anti-fungal activity against barley powdery mildew and cucumber powdery mildew, more efficiently in the latter case than treatments with fenarimol and polyoxin B.[4]
It reacts with KOH to form a deep, reddish-magenta compound.
Effect on human cancer cells
Also found in rhubarb,[5] the orange compound appears to have potential to suppress 6-phosphogluconate dehydrogenase, or 6PGD. 6PGD is the third enzyme of the pentose phosphate pathway, or PPP, an oxidative process fueling growth in a still-relatively-unknown way. But it appears that arresting the chemical machinery at its third step could be promising for oncology. The parietin, identified from an FDA database of 2,000 known suppressors of 6PGD, killed half the human leukemia cells over two days in the laboratory. The pigment also slowed the growth of other human cancer cells in mouse models, according to the study. A more-potent derivative of the parietin called S3 may even cut the growth of lung cancer cells implanted in mice by two-thirds, over the course of 11 days. The compound also appears to be non-toxic to healthy cells.[6][7]
References
- ↑ "Xanthoria parietina is a widespread lichen coloured by the orange cortical pigment parietin (= physcion). We studied the pigment content in 60 thalli sampled in 4 habitats along a sun–shade gradient from evergreen boreal forests through open deciduous stands to sea cliffs. The significant positive regression between contents of parietin per unit area and site factors (reflecting the openness of the canopy relative to an open sky) across sampled habitats suggested a photoprotective role of parietin at UV-B and/or blue wavelengths, the two absorbance maxima of parietin. UV-B susceptibility of X. parietina, measured as permanent reductions in photosystem II, decreased highly significantly with increasing parietin content per thallus area. However, as much as three-fold greater UV-B irradiances than ambient daily summer maxima, maintained continuously for 240 h were required to cause UV-B damage even in thalli of shaded habitats. Since a previous study has documented a high PAR susceptibility of parietin-deficient X. parietina in the absence of UV-B, there are reasons to believe that the blue light screening of parietin is functionally more important than the UV-B screening. A strong positive relationship between parietin content per unit area and reflectance at 500 nm allows the parietin content in X. parietina thalli to be assessed non-destructively by reflectance measurements" Gauslaa Yngvar, Margrete Ustvedt Elin (2003). "Is parietin a UV-B or a blue-light screening pigment in the lichen Xanthoria parietina?". Photochem. Photobiol. Sci. 2 (4): 424–432. doi:10.1039/b212532c. PMID 12760542.
- ↑ "This study reports UV screening pigments in the upper cortices of two widespread lichens collected in three sun-exposed locations along a latitudinal gradient from the Arctic lowland to alpine sites of the Central European Alps. Populations from the Alps receive 3–5 times higher UV-B irradiance than their Arctic counterparts from Svalbard because of latitudinal and altitudinal gradients in UV-B irradiance.... This implies that Arctic populations maintain a high level of screening pigments in spite of low ambient UV-B, and that the studied lichen species presumably may tolerate an increase in UV-B radiation due to the predicted thinning of the ozone layer over polar areas" Nybakken Line; Asbjørn Solhaug Knut; Bilger Wolfgang; Gauslaa Yngvar (2004). "The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats". Oecologia 140 (2): 211–216. doi:10.1007/s00442-004-1583-6. PMID 15138881. Bibcode: 2004Oecol.140..211N.
- ↑ Asbjorn Solhaug Knut (2003). "UV-induction of sun-screening pigments in lichens". New Phytologist 158 (1): 91–100. doi:10.1046/j.1469-8137.2003.00708.x.
- ↑ Gyung Ja Choi; Seon-Woo Lee; Kyoung Soo Jang; Jin-Seog Kim; Kwang Yun Cho; Jin-Cheol Kim (December 2004). "Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews". Crop Protection 23 (12): 1215–1221. doi:10.1016/j.cropro.2004.05.005.
- ↑ "Cancer Growth Could be Slowed by Little-known Pigment in Rhubarb". 2015-10-22. http://www.laboratoryequipment.com/news/2015/10/cancer-growth-could-be-slowed-little-known-pigment-rhubarb?et_cid=4898757&et_rid=599618518&location=top.
- ↑ "Orange lichens are source for potential anticancer drug". 2015-10-21. http://news.emory.edu/stories/2015/10/winship_chen_pigment/campus.html.
- ↑ Basile, Adriana; Rigano, Daniela; Loppi, Stefano; Di Santi, Annalisa; Nebbioso, Angela; Sorbo, Sergio; Conte, Barbara; Paoli, Luca et al. (2015-04-09). "Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin". International Journal of Molecular Sciences 16 (4): 7861–7875. doi:10.3390/ijms16047861. ISSN 1422-0067. PMID 25860944.
- Caloplaca coralloides chemistry
- Edwards, Howell G. M.; Emma M. Newton; David D. Wynn-Williams; Steven R. Coombes (2003-03-12). "Molecular spectroscopic studies of lichen substances 1: parietin and emodin". Journal of Molecular Structure 648 (1–2): 49–59. doi:10.1016/S0022-2860(02)00384-8. Bibcode: 2003JMoSt.648...49E.
- Solhaug, Knut A.; Yngvar Gauslaa (November 1996). "Parietin, a photoprotective secondary product of the lichen Xanthoria parietina". Oecologia 108 (3): 412–418. doi:10.1007/BF00333715. PMID 28307855. Bibcode: 1996Oecol.108..412S.
 |
