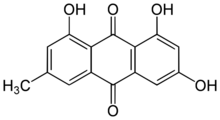
Chemistry:Emodin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,8-Trihydroxy-6-methylanthracene-9,10-dione | |
| Other names
6-Methyl-1,3,8-trihydroxyanthraquinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Appearance | Orange solid |
| Density | 1.583±0.06 g/cm3 |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is a chemical compound, of the anthraquinone family, that can be isolated from rhubarb, buckthorn, and Japanese knotweed (Reynoutria japonica syn. Polygonum cuspidatum).[1] Emodin is particularly abundant in the roots of the Chinese rhubarb (Rheum palmatum), knotweed and knotgrass (Polygonum cuspidatum and multiflorum) as well as Hawaii ‘au‘auko‘i cassia seeds or coffee weed (Semen cassia).[2] It is specifically isolated from Rheum palmatum L.[3] It is also produced by many species of fungi, including members of the genera Aspergillus, Pyrenochaeta, and Pestalotiopsis, inter alia. The common name is derived from Rheum emodi, a taxonomic synonym of Rheum australe, (Himalayan rhubarb) and synonyms include emodol, frangula emodin, rheum emodin, 3-methyl-1,6,8-trihydroxyanthraquinone, Schüttgelb (Schuttgelb), and Persian Berry Lake.[4]
Pharmacology
Emodin is an active component of several plants used in Traditional Chinese Medicine (TCM) such as Rheum palmatum, Polygonum cuspidatum and Polygonum multiflorum. It has various actions including laxative, antibacterial and antiinflammatory effects,[5][6] and has also been identified as having potential antiviral activity against coronaviruses such as SARS-CoV-2,[7][8] being one of the major active components of the antiviral TCM formulation Lianhua Qingwen.[9][10]
A computational study was conducted to investigate the inhibition mechanism on the formation of the Spike-ACE2 protein complex.[2] Specifically, it is seen dose-dependent inhibition in the prevention of infection by the SARS-Cov-1 whose evidence has stimulated further investigations on SARS-Cov-2.[2]
Emodin has been shown to inhibit the ion channel of protein 3a which could play a crucial role in the release of the virus from infected cells.[11]
List of species
The following plant species produce emodin:
- Acalypha australis[12]
- Cassia occidentalis[13]
- Cassia siamea[14]
- Frangula alnus[15]
- Glossostemon bruguieri[16]
- Kalimeris indica[17]
- Polygonum hypoleucum[18]
- Reynoutria japonica (syn. Fallopia japonica)[19] (syn. Polygonum cuspidatum[20])
- Rhamnus alnifolia, the alderleaf buckthorn[21]
- Rhamnus cathartica, the common buckthorn[21]
- Rheum palmatum[22]
- Rumex nepalensis[23]
- Senna obtusifolia[24] (syn. Cassia obtusifolia[25])
- Thielavia subthermophila[26]
- Ventilago madraspatana[27]
Emodin also occurs in variable amounts in members of the crustose lichen genus Catenarina.[28]
Compendial status
- British Pharmacopoeia[29]
- List of compounds with carbon number 15
References
- ↑ Dorland's Medical Dictionary (1938)
- ↑ 2.0 2.1 2.2 "Preventing the Interaction between Coronaviruses Spike Protein and Angiotensin I Converting Enzyme 2: An in Silico Mechanistic Case Study on Emodin as a Potential Model Compound". Applied Sciences 10 (18): 6358. 2020. doi:10.3390/app10186358.
- ↑ Medicinal Plants – Recent Advances in Research and Development. Singapore: Springer Singapore. 3 November 2017. pp. 339. ISBN 978-981-10-5978-0. https://books.google.com/books?id=D-Q8DwAAQBAJ.
- ↑ CID 3220 from PubChem
- ↑ "Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics". Phytotherapy Research 30 (8): 1207–18. August 2016. doi:10.1002/ptr.5631. PMID 27188216.
- ↑ "Emodin and Its Role in Chronic Diseases". Anti-inflammatory Nutraceuticals and Chronic Diseases. Advances in Experimental Medicine and Biology. 928. 2016. pp. 47–73. doi:10.1007/978-3-319-41334-1_3. ISBN 978-3-319-41332-7.
- ↑ "Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction". Antiviral Research 74 (2): 92–101. May 2007. doi:10.1016/j.antiviral.2006.04.014. PMID 16730806.
- ↑ "Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2". Cell Discovery 6: 14. 2020. doi:10.1038/s41421-020-0153-3. PMID 32194980.
- ↑ "A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components". Molecular BioSystems 12 (2): 606–13. February 2016. doi:10.1039/c5mb00448a. PMID 26687282.
- ↑ "Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2)". Pharmacological Research 156: 104761. June 2020. doi:10.1016/j.phrs.2020.104761. PMID 32205232.
- ↑ "Emodin inhibits current through SARS-associated coronavirus 3a protein". Antiviral Research 90 (1): 64–9. April 2011. doi:10.1016/j.antiviral.2011.02.008. PMID 21356245.
- ↑ "[Chemical constituents of aerial part of Acalypha australis]" (in Chinese). Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica 33 (12): 1415–7. June 2008. PMID 18837345.
- ↑ "Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile". Fitoterapia 81 (4): 223–30. June 2010. doi:10.1016/j.fitote.2009.09.008. PMID 19796670.
- ↑ "Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts". Journal of Ethnopharmacology 127 (1): 108–11. January 2010. doi:10.1016/j.jep.2009.09.040. PMID 19799981.
- ↑ "Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark". Food Chemistry 131 (4): 1174–1180. April 2012. doi:10.1016/j.foodchem.2011.09.094. https://www.sciencedirect.com/science/article/abs/pii/S0308814611013719.
- ↑ "Constituents from Moghat, the Roots of Glossostemon bruguieri (Desf.)". Molecules 8 (8): 614–621. August 2003. doi:10.3390/80800614.
- ↑ "[Studies on the chemical constituents of Kalimeris indica]" (in Chinese). Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials 33 (4): 551–4. April 2010. PMID 20845783.
- ↑ "The metabolic benefits of Polygonum hypoleucum Ohwi in HepG2 cells and Wistar rats under lipogenic stress". Journal of Agricultural and Food Chemistry 58 (8): 5174–80. April 2010. doi:10.1021/jf100046h. PMID 20230058. http://ntur.lib.ntu.edu.tw/bitstream/246246/239385/-1/371.pdf.
- ↑ "Reynoutria japonica (Polygonaceae)". Dr. Duke's Phytochemical and Ethnobotanical Databases. U.S. Department of Agriculture. https://phytochem.nal.usda.gov/phytochem/ethnoPlants/show/6292?et=.
- ↑ "Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci". Fitoterapia 81 (1): 30–4. January 2010. doi:10.1016/j.fitote.2009.06.019. PMID 19616082.
- ↑ 21.0 21.1 Sacerdote, Allison B.; King, Richard B. (2014). "Direct Effects of an Invasive European Buckthorn Metabolite on Embryo Survival and Development in Xenopus laevis and Pseudacris triseriata". Journal of Herpetology 48 (1): 51–58. doi:10.1670/12-066. http://www.cnah.org/pdf/88516.pdf.
- ↑ "Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer". Oncology Reports 26 (1): 81–9. July 2011. doi:10.3892/or.2011.1257. PMID 21491088.
- ↑ "Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumex nepalensis". Planta Medica 76 (14): 1564–9. October 2010. doi:10.1055/s-0030-1249779. PMID 20379952.
- ↑ "Senna obtusifolia (Fabaceae)". Dr. Duke's Phytochemical and Ethnobotanical Databases. U.S. Department of Agriculture. https://phytochem.nal.usda.gov/phytochem/plants/show/1815?et=.
- ↑ "Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species". Journal of Agricultural and Food Chemistry 51 (26): 7629–31. December 2003. doi:10.1021/jf034727t. PMID 14664519.
- ↑ "Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis". Journal of Natural Products 72 (10): 1825–35. October 2009. doi:10.1021/np9002977. PMID 19746917.
- ↑ "Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn". The Journal of Pharmacy and Pharmacology 62 (9): 1158–66. September 2010. doi:10.1111/j.2042-7158.2010.01151.x. PMID 20796195.
- ↑ Søchting, Ulrik; Søgaard, Majbrit Zeuthen; Elix, John A.; Arup, Ulf; Elvebakk, Arve; sancho, Leopoldo G. (2014). "Catenarina (Teloschistaceae, Ascomycota), a new Southern Hemisphere genus with 7-chlorocatenarin". The Lichenologist 46 (2): 175–187. doi:10.1017/s002428291300087x.
- ↑ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf.
 |
