Chemistry:Paxilline

| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,4bS,6aS,12bS,12cR,14aS)-4b-Hydroxy-2-(2-hydroxypropan-2-yl)-12b,12c-dimethyl-5,6,6a,7,12,12b,12c,13,14,14a-decahydro-2H-[1]benzopyrano[5′,6′:6,7]indeno[1,2-b]indol-3(4bH)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Paxilline |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H33NO4 | |
| Molar mass | 435.56 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by Penicillium paxilli which was first characterized in 1975.[1][2] Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic.[3]
Paxilline was found to significantly extend the lifespan, healthspan, and mobility of aged C. elegans worms, but had no such effect on young worms.[4] Paxilline was not found to induce seizures when injected intracerebroventricularly in mice[5] but paradoxically had anticonvulsant activity against picrotoxin and pentylenetetrazol seizures in mice.[6] It has also been used in mice to induce autism-like behaviors through inhibition of the BK channel.[7]
Biosynthesis
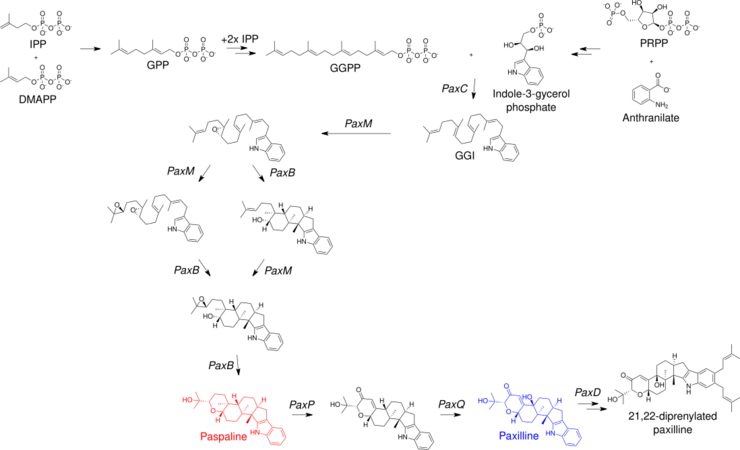
Paxiline biosynthesis starts with the synthesis of geranylgeranyl pyrophosphate via the terpenoid pathway and indole-3-glycerol phosphate, which is an intermediate in the tryptophan biosynthesis pathway.[8] By expressing six genes known to be necessary for Paxilline synthesis in Aspergillus oryzae, the further steps in the biosynthesis were identified; two epoxidations and two cyclizations yield paspaline, then two oxidation reactions and a demethylation complete the synthesis.[9] This biosynthesis is notable for its unusual stereospecific polycyclization mechanism that has not been replicated in a chemical synthesis, though other mechanisms have been devised for total synthesis of Paxilline.[10] Paxilline has also been found to be mono- or di-prenylated with DMAPP by an atypical prenyltransferase enzyme.[11]
Sources and references
- ↑ Paxilline product page from Fermentek
- ↑ "The structure of paxilline, a tremorgenic metabolite of penicillium paxilli bainier" (in en). Tetrahedron Letters 16 (30): 2531–2534. 1975-01-01. doi:10.1016/S0040-4039(00)75170-7. ISSN 0040-4039. https://www.sciencedirect.com/science/article/abs/pii/S0040403900751707.
- ↑ Evans, Tim J.; Gupta, Ramesh C. (2018-01-01). "Tremorgenic Mycotoxins" (in en). Veterinary Toxicology: 1033–1041. doi:10.1016/B978-0-12-811410-0.00074-X. ISBN 9780128114100. https://www.sciencedirect.com/science/article/pii/B978012811410000074X.
- ↑ Li, Guang; Gong, Jianke; Liu, Jie; Liu, Jinzhi; Li, Huahua; Hsu, Ao-Lin; Liu, Jianfeng; Xu, X.Z. Shawn (2019). "Genetic and pharmacological interventions in the aging motor nervous system slow motor aging and extend life span in C. Elegans". Science Advances 5 (1): eaau5041. doi:10.1126/sciadv.aau5041. PMID 30613772.
- ↑ Juhng, KN; Kokate, TG; Yamaguchi, S; Kim, BY; Rogowski, RS; Blaustein, MP; Rogawski, MA (1999). "Induction of seizures by the potent K+ channel-blocking scorpion venom peptide toxins tityustoxin-K(alpha) and pandinustoxin-K(alpha)". Epilepsy Res 34 (2–3): 177–86. doi:10.1016/S0920-1211(98)00111-9. PMID 10210033.
- ↑ Sheehan, JJ; Benedetti, BL; Barth, AL (2009). "Anticonvulsant effects of the BK-channel antagonist paxilline". Epilepsia 50 (4): 711–20. doi:10.1111/j.1528-1167.2008.01888.x. PMID 19054419.
- ↑ Fyke, William; Alarcon, Juan M.; Velinov, Milen; Chadman, Kathryn K. (2021). "Pharmacological inhibition of BKCa channels induces a specific social deficit in adult C57BL6/J mice". Behavioral Neuroscience 135 (4): 462–468. doi:10.1037/bne0000459. PMID 33734729. https://doi.org/10.1037/bne0000459.
- ↑ Fueki, Shuhei; Tokiwano, Tetsuo; Toshima, Hiroaki; Oikawa, Hideaki (2004-08-01). "Biosynthesis of Indole Diterpenes, Emindole, and Paxilline: Involvement of a Common Intermediate". Organic Letters 6 (16): 2697–2700. doi:10.1021/ol049115o. ISSN 1523-7060. PMID 15281747. https://doi.org/10.1021/ol049115o.
- ↑ Tagami, Koichi; Liu, Chengwei; Minami, Atsushi; Noike, Motoyoshi; Isaka, Tetsuya; Fueki, Shuhei; Shichijo, Yoshihiro; Toshima, Hiroaki et al. (2013-01-30). "Reconstitution of Biosynthetic Machinery for Indole-Diterpene Paxilline in Aspergillus oryzae". Journal of the American Chemical Society 135 (4): 1260–1263. doi:10.1021/ja3116636. ISSN 0002-7863. PMID 23311903. https://doi.org/10.1021/ja3116636.
- ↑ Thomas, William P.; Pronin, Sergey V. (2021-03-16). "New Methods and Strategies in the Synthesis of Terpenoid Natural Products". Accounts of Chemical Research 54 (6): 1347–1359. doi:10.1021/acs.accounts.0c00809. ISSN 0001-4842. PMID 33596652. PMC 10122273. https://doi.org/10.1021/acs.accounts.0c00809.
- ↑ Liu, Chengwei; Noike, Motoyoshi; Minami, Atsushi; Oikawa, Hideaki; Dairi, Tohru (2014-01-01). "Functional analysis of a prenyltransferase gene (paxD) in the paxilline biosynthetic gene cluster" (in en). Applied Microbiology and Biotechnology 98 (1): 199–206. doi:10.1007/s00253-013-4834-9. ISSN 1432-0614. PMID 23525886. https://doi.org/10.1007/s00253-013-4834-9.
 |