Chemistry:Pentamethylmolybdenum
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C5H15Mo | |
| Molar mass | 171.13 g·mol−1 |
| Appearance | turquoise blue crystals |
| Boiling point | decomposes at −10°C |
| Structure[1] | |
| tetragonal | |
| I4 | |
a = 7.680, b = 7.680, c = 6.490
| |
Lattice volume (V)
|
382.80 |
Formula units (Z)
|
2 |
| Related compounds | |
Related compounds
|
Pentamethylarsenic Pentamethylbismuth Pentamethylantimony pentamethyltantalum Hexamethylmolybdenum |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
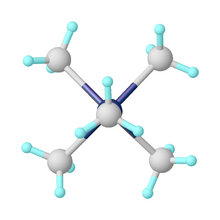
Pentamethylmolybdenum is an organomolybdenum compound containing five methyl groups bound to a central molybdenum atom.[2] The shape of the molecule is a square pyramid.[3]
The molecule is similar to pentamethyltungsten in shape and properties.[3]
Production
Pentamethylmolybdenum can be prepared from molybdenum pentachloride and dimethyl zinc at low temperature between −70 and −20. Another possible creation route, is from molybdenum oxychloride.[2] Pentamethylmolybdenum is paramagnetic with one unpaired electron. The character of this electron is two thirds 4dz2 and one third 4dx2−y2.[2]
Properties
Pentamethylmolybdenum is unstable and sensitive to oxygen. It turns black when exposed to air, or heated over −10°C.[2] The Raman spectrum has bands at 1181, 960, 90, 882, 783, 672, 620, 565, 523, 507, 451, 366, 308, 267 and 167 cm−1.[2]
References
- ↑ Vreshch V. (2018). "Crystal Structure of Pentamethylmolybdenum(V)" (in en). http://crystallography-online.com/structure/7110877.
- ↑ 2.0 2.1 2.2 2.3 2.4 Roessler, Beatrice; Kleinhenz, Sven; Seppelt, Konrad (2000). "Pentamethylmolybdenum". Chemical Communications (12): 1039–1040. doi:10.1039/b000987n.
- ↑ 3.0 3.1 Werner, Helmut (2008) (in en). Landmarks in Organo-Transition Metal Chemistry: A Personal View. Springer Science & Business Media. p. 309. ISBN 9780387098487. https://books.google.com/books?id=dP4LTfaPzAMC&pg=PA309.
 |
