Physics:Square pyramidal molecular geometry
| Square pyramidal molecular geometry | |
|---|---|
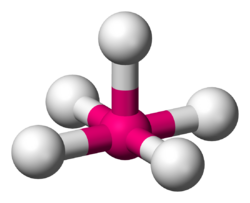 | |
| Examples | Chlorine pentafluoride (ClF 5), MnCl2− 5 |
| Point group | C4v |
| Coordination number | 5 |
Square pyramidal geometry describes the shape of certain chemical compounds with the formula ML
5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably [Ni(CN)
5]3−.[1]
As a transition state in Berry pseudorotation
As a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square pyramidal geometry. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid.
Pseudorotation also occurs in square pyramidal molecules. Molecules with this geometry, as opposed to trigonal bipyramidal, exhibit heavier vibration. The mechanism used is similar to the Berry mechanism.
Examples
Some molecular compounds that adopt square pyramidal geometry are XeOF4,[2] and various halogen pentafluorides (XF5, where X = Cl, Br, I).[3][4] Complexes of vanadium(IV), such as vanadyl acetylacetonate, [VO(acac)2], are square pyramidal (acac = acetylacetonate, the deprotonated anion of acetylacetone (2,4-pentanedione)).
See also
- AXE method
- Square pyramid
- Hypervalent molecule
- Molecular geometry
References
- ↑ Spiro, Thomas G.; Terzis, Aristides; Raymond, Kenneth N. (1970). "Structure of Ni(CN)3−5. Raman, infrared, and x-ray crystallographic evidence". Inorg. Chem. 9 (11): 2415. doi:10.1021/ic50093a006.
- ↑ "Square Pyramidal Molecular Geometry. VSEPR". Archived from the original on 2009-11-02. https://web.archive.org/web/20091102023419/http://www.up.ac.za/academic/chem/mol_geom/planpyr.htm.
- ↑ "Square Pyramidal Geometry". http://intro.chem.okstate.edu/1314F97/Chapter9/5BP1LP.html.
- ↑ Miessler, G. L.; Tarr, D. A. (2004). Inorganic Chemistry (3rd ed.). Pearson/Prentice Hall. ISBN 0-13-035471-6. https://archive.org/details/inorganicchemist03edmies.
External links
- Chem| Chemistry, Structures, and 3D Molecules[yes|permanent dead link|dead link}}]
- Indiana University Molecular Structure Center
- Interactive molecular examples for point groups
- Molecular Modeling
- Animated Trigonal Planar Visual
 |
