Chemistry:Pentamethyltantalum
| |
| Names | |
|---|---|
| Systematic IUPAC name
pentamethyl-λ5-tantalane | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C5H15Ta | |
| Molar mass | 256.123 g·mol−1 |
| Appearance | yellow oil, green solid at −20° |
| Melting point | 0 °C (32 °F; 273 K)[1] |
| Boiling point | decomposes above 25° to methane |
| Solubility | ether, pentane, 2-methylbutane |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
169.8[2] 213 kJ/mol[3] |
| Related compounds | |
Related compounds
|
Pentamethylarsenic Pentamethylbismuth Pentamethylantimony pentabenzyltantalum |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted.[4] Its discovery was part of a sequence that lead to Richard R. Schrock's Nobel Prize discovery in olefin metathesis.[5]
Production
Pentamethyltantalum can be made from the reaction of methyllithium with Ta(CH3)3Cl2.[6] Ta(CH3)3Cl2 is in turn made from tantalum pentachloride and dimethylzinc.[7]
The preparation was inspired by the existence of pentaalkyl compounds of phosphorus and arsenic, and the discovery of hexamethyltungsten. The discoverer, Richard R. Schrock considered tantalum to be a metallic phosphorus, and tried the use of methyllithium.[8]
Properties
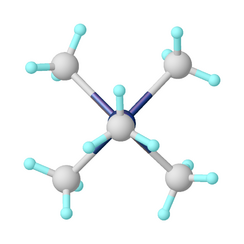
The pentamethyltantalum adopts a square pyramid shape. Ignoring the C-H bonds, the molecule has C4v symmetry. The four carbon atoms at the base of the pyramid are called basal, and the carbon atom at the top is called apical or apex. The distance from tantalum to the apical carbon atom is 2.11 Å, and to the basal carbon atoms is 2.180 Å. The distance from hydrogen to carbon in the methyl groups is 1.106 Å. The angle subtended by two basal carbon bonds is 82.2°, and the angle between the bonds to the apex and a carbon on the base is about 111.7°.[9][10]
At room temperature pentamethyltantalum can spontaneously explode, so samples are stored below 0°.[10]
Reactions
With many carbon-hydrogen bonds near Ta, analogues of pentamethyltantalum are susceptible to alpha elimination.[5]
Excess methyllithium reacts to yield higher coordinated methyl tantalum ions [Ta(CH3)6]− and [Ta(CH3)7]2−.[6]
Pentamethyltantalum in solution forms stable insoluble complex material when mixed with dmpe (CH3)2PCH2CH2P(CH3)2.[6]
With nitric oxide it gives a white coloured dimer with formula {TaMe3[ON(Me)NO]2}2 (Me=CH3).[11]
References
- ↑ Yaws, Carl L. (2015) (in en). The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics. Gulf Professional Publishing. p. 87. ISBN 9780128011461. https://books.google.com/books?id=GutDBAAAQBAJ&pg=PA87.
- ↑ Adedeji, Festus A.; Connor, Joseph A.; Skinner, Henry A.; Galyer, Lee; Wilkinson, Geoffrey (1976). "Heat of formation of pentamethylantalum and hexamethyltungsten". Journal of the Chemical Society, Chemical Communications (5): 159. doi:10.1039/C39760000159.
- ↑ "Pentamethyl tantalum" (in en). http://webbook.nist.gov/cgi/cbook.cgi?ID=C53378726&Mask=1#Thermo-Gas.
- ↑ Urben, Peter (2013) (in en). Bretherick's Handbook of Reactive Chemical Hazards. Academic Press. p. 744. ISBN 9780080523408. https://books.google.com/books?id=Hda6Nv1SMg4C&pg=PA744.
- ↑ 5.0 5.1 Schrock, Richard R. (8 December 2005). "Multiple Metal-Carbon Bonds for Catalytic Metathesis Reactions Nobel Lecture". Angewandte Chemie International Edition in English 45 (23): 3748–59. doi:10.1002/anie.200600085. PMID 16703641. https://www.nobelprize.org/nobel_prizes/chemistry/laureates/2005/schrock-lecture.pdf. Retrieved 18 June 2017.
- ↑ 6.0 6.1 6.2 Schrock, R. R.; Meakin, P. (August 1974). "Pentamethyl complexes of niobium and tantalum". Journal of the American Chemical Society 96 (16): 5288–5290. doi:10.1021/ja00823a064.
- ↑ Herrmann, W. A. (2014) (in de). Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 7, 1997: Volume 7: Transition Metals. Georg Thieme Verlag. pp. 160–161. ISBN 9783131794710. https://books.google.com/books?id=64yZAwAAQBAJ&pg=PT160.
- ↑ Schrock, Richard R. (2002). "The Discovery and Development of High Oxidation State Alkilidene Complexes". in Bertrand, Guy (in en). Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents. CRC Press. pp. 206–208. ISBN 9780203910924. https://books.google.com/books?id=ElV85eHbubQC&pg=PA207.
- ↑ Albright, Thomas A.; Tang, Huang (November 1992). "The Structure of Pentamethyltantalum". Angewandte Chemie International Edition in English 31 (11): 1462–1464. doi:10.1002/anie.199214621.
- ↑ 10.0 10.1 Haaland, Arne; Hammel, Andreas; Rypdal, Kristin; Verne, Hans Peter; Volden, Hans Vidar; Pulham, Colin (November 1992). "The Structures of Pentamethyltantalum and -Antimony: One Square Pyramid and One Trigonal Bipyramid". Angewandte Chemie International Edition in English 31 (11): 1464–1467. doi:10.1002/anie.199214641.
- ↑ Middleton, A. Robert; Wilkinson, Geoffrey (1980). "Interaction of nitric oxide with paramagnetic and diamagnetic alkyls of titanium, zirconium, vanadium, niobium, and tantalum". Journal of the Chemical Society, Dalton Transactions (10): 1888. doi:10.1039/DT9800001888.
 |

