Chemistry:Peroxybenzoic acid
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzenecarboperoxoic acid[1] | |||
| Systematic IUPAC name
Oxobenzoic acid Oxidobenzoic acid | |||
| Other names
Peroxybenzoic acid
Perbenzoic acid Benzoperoxoic acid Hydroxy benzoate Benzoyl hydroperoxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | C017611 | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.12 g/mol | ||
| Melting point | 41 to 42 °C (106 to 108 °F; 314 to 315 K)[2] | ||
| Acidity (pKa) | 7.8[2] | ||
| Related compounds | |||
Related compounds
|
m-Chloroperoxybenzoic acid Hydrogen peroxide Benzoic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
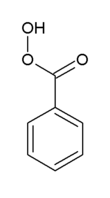
Peroxybenzoic acid is an organic compound with the formula C6H5CO3H. It is the simplest aryl peroxy acid. It may be synthesized from benzoic acid and hydrogen peroxide,[3] or by the treatment of benzoyl peroxide with sodium methoxide, followed by acidification.[4]
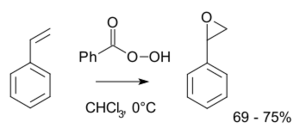
Like other peroxyacids, it may be used to generate epoxides, such as styrene oxide from styrene:[5]
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 749, 761. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 Elvers, B. et al. (ed.) (1991) Ullmann's Encyclopedia of Industrial Chemistry, 5th ed. Vol. A19, Wiley, p. 206
- ↑ Silbert, L. S.; Siegel, E.; Swern, D. (1964). "Peroxybenzoic Acid". Org. Synth. 44: 81. doi:10.15227/orgsyn.044.0081.
- ↑ Géza Braun (1928). "Perbenzoic Acid". Org. Synth. 8: 30. doi:10.15227/orgsyn.008.0030.
- ↑ Harold Hibbert and Pauline Burt (1941). "Styrene Oxide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0494.; Collective Volume, 1, pp. 494
 |