Chemistry:Styrene oxide
| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenyloxirane | |
| Other names
Epoxystyrene; Styryl oxide; Phenylethylene oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| 108582 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 50213 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 3082 |
| |
| |
| Properties | |
| C8H8O | |
| Molar mass | 120.151 g·mol−1 |
| Appearance | Colorless to light yellow liquid |
| Density | 1.052 g/mL |
| Melting point | −37 °C (−35 °F; 236 K) |
| Boiling point | 194 °C (381 °F; 467 K) |
| Hazards | |
| Safety data sheet | Oxford University MSDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H312, H319, H350 | |
| P201, P202, P264, P280, P281, P302+352, P305+351+338, P308+313, P312, P322, P337+313, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
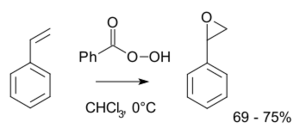
Styrene oxide is an epoxide derived from styrene. It can be prepared by epoxidation of styrene with peroxybenzoic acid, in the Prilezhaev reaction:[1]
Styrene oxide is slightly soluble in water. A trace amount of acid in water causes hydrolysis to racemic phenylethyleneglycol via a benzylic cation. If the amount of water is not sufficient, acid-catalyzed isomerization for phenylacetaldehyde will occur.[2]
Styrene oxide in the body is metabolized to mandelic acid, phenylglyoxylic acid, benzoic acid and hippuric acid.
Hydrogenation of styrene oxide affords phenethyl alcohol.[3]
Stereospecific reactions
Since styrene oxide has a chiral center at the benzylic carbon atom, there are (R)-styrene oxide and (S)-styrene oxide. If optically pure reagent is used, only one optically pure compound will be obtained.
Toxicology
Styrene oxide is a main metabolite of styrene in humans or animals, resulting from oxidation by cytochrome P450. It is considered possibly carcinogenic from gavaging significant amounts into mice and rats.[4] Styrene oxide is subsequently hydrolyzed in vivo to styrene glycol by epoxide hydrolase.[5]
Styrene oxide has a chiral center and thus two enantiomers. It has been reported that the two enantiomers had different toxicokinetics and toxicity[citation needed]. It was reported that the (R)-styrene oxide was preferentially formed in mice, especially in the lung, whereas the (S)-styrene oxide was preferentially generated in rats. In human volunteers, the cumulative excretion of the (S)-enantiomer of styrene glycol and mandelic acid were higher than the R form after exposure to styrene. In human liver microsomes, cytochrome P450-mediated styrene oxidation showed the production of more S enantiomer relative to the R enantiomer. It was also found that (S)-styrene oxide was preferentially hydrolyzed than the R enantiomer in human liver microsomes. Animal studies have shown that the (R)-enantiomer of styrene oxide was more toxic than the (S)-enantiomer in mice.
References
- ↑ Harold Hibbert and Pauline Burt (1941). "Styrene Oxide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0494.; Collective Volume, 1, pp. 494
- ↑ Verfahren zur Herstellung von Phenylacetaldehyde, BASF-Patent DE3546372A1 vom 2. Juli 1987
- ↑ Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- ↑ EPA Styrene Oxide evaluation
- ↑ Kenneth C. Liebman (1975). "Metabolism and toxicity of styrene". Environmental Health Perspectives 11: 115–119. doi:10.2307/3428333. PMID 809262. PMC 1475194. http://ehis.niehs.nih.gov/members/1975/011/11018.PDF.[no|permanent dead link|dead link}}]
 |