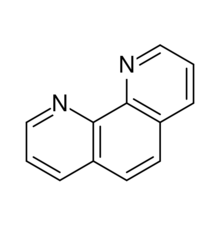
Chemistry:Phenanthroline
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,10-Phenanthroline[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 126461 | |
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 4040 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 2811 |
| |
| |
| Properties | |
| C12H8N2 | |
| Molar mass | 180.21 g/mol |
| Appearance | colourless crystals |
| Density | 1.31 g/cm3 |
| Melting point | 118.56 °C (245.41 °F; 391.71 K)[2] |
| Boiling point | 409.2 [2] |
| high[2] | |
| Solubility in other solvents | acetone, ethanol[2] |
| Acidity (pKa) | 4.84 (phenH+)[3] |
| Hazards | |
| Main hazards | mild neurotoxin, strong nephrotoxin, and powerful diuretic |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H400, H410 | |
| P264, P270, P273, P301+310, P321, P330, P391, P405, P501 | |
| Related compounds | |
Related compounds
|
2,2'-bipyridine ferroin phenanthrene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, forming strong complexes with most metal ions.[4][5] It is often sold as the monohydrate.
Synthesis
Phenanthroline may be prepared by two successive Skraup reactions of glycerol with o-phenylenediamine, catalyzed by sulfuric acid, and an oxidizing agent, traditionally aqueous arsenic acid or nitrobenzene.[6] Dehydration of glycerol gives acrolein which condenses with the amine followed by a cyclization.
Coordination chemistry
In terms of its coordination properties, phenanthroline is similar to 2,2'-bipyridine (bipy) but binds metals more tightly since the chelating nitrogen donors are preorganized. Phenanthroline is however a weaker donor than bipy.[7]
Many homoleptic complexes are known. Particularly well studied is [Fe(phen)3]2+, called "ferroin." It was used for the photometric determination of Fe(II).[8] It is used as a redox indicator with standard potential +1.06 V. The reduced ferrous form has a deep red colour and the oxidised form is light-blue.[9] The pink complex [Ni(phen)3]2+ has been resolved into its Δ and Λ isomers.[10] Copper(I) forms [Cu(phen)2]+, which is luminescent.[11][12]
Bioinorganic chemistry
The ferroin analogue [Ru(phen)3]2+ has long been known to be bioactive.[13]
1,10-Phenanthroline is an inhibitor of metallopeptidases, with one of the first observed instances reported in carboxypeptidase A.[14] Inhibition of the enzyme occurs by removal and chelation of the metal ion required for catalytic activity, leaving an inactive apoenzyme. 1,10-Phenanthroline targets mainly zinc metallopeptidases, with a much lower affinity for calcium.[15]
Related phen ligands
A variety of substituted derivatives of phen have been examined as ligands.[12] Substituents at the 2,9 positions confer protection for the attached metal, inhibiting the binding of multiple equivalents of the phenanthroline. Phen itself form complexes of the type [M(phen)3]Cl2 when treated with metal dihalides (M = Fe, Co, Ni). By contrast, neocuproine and bathocuproine form 1:1 complexes such as [Ni(neo/batho-cuproine)Cl2]2.[16]
| ligand | pKa | comment/alt. name | illustration |
|---|---|---|---|
| 1,10-phenanthroline | 4.86 | phen |  |
| 2,2'-bipyridine | 4.30 | less basic than phen | |
| 5-nitro-1,10-phenanthroline | 3.57 | ||
| 2,9-dimethyl-1,10-phenanthroline | unknown | neocuproine | |
| 4,7-dimethyl-1,10-phenanthroline | 5.97 | ||
| 4,7-diphenyl-1,10-phenanthroline | unknown | bathophenanthroline | |
| 5,6-dimethyl-1,10-phenanthroline | 5.20 | ||
| 3,4,7,8-tetramethylphenanthroline | 6.31 | 3,4,7,8-Me4phen | |
| 4,7-dimethoxy-1,10‐phenanthroline | 6.45 | 4,7-(MeO)2phen[18] |
As an indicator for alkyllithium reagents
Alkyllithium reagents form deeply colored derivatives with phenanthroline. The alkyllithium content of solutions can be determined by treatment of such reagents with small amounts of phenanthroline (ca. 1 mg) followed by titration with alcohols to a colourless endpoint.[19] Grignard reagents may be similarly titrated.[20]
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 211. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 Haynes, p. 3.444
- ↑ Haynes, p. 5.95
- ↑ Luman, C.R. and Castellano, F.N. (2003) "Phenanthroline Ligands" in Comprehensive Coordination Chemistry II. Elsevier. ISBN:978-0-08-043748-4.
- ↑ Sammes, Peter G.; Yahioglu, Gokhan (1994). "1,10-Phenanthroline: A versatile ligand". Chemical Society Reviews 23 (5): 327. doi:10.1039/cs9942300327.
- ↑ Halcrow, Barbara E.; Kermack, William O. (1946). "43. Attempts to find new antimalarials. Part XXIV. Derivatives of o-phenanthroline (7 : 8 : 3′ : 2′-pyridoquinoline)". J. Chem. Soc.: 155–157. doi:10.1039/jr9460000155. PMID 20983293.
- ↑ Teng, Qiaoqiao; Huynh, Han Vinh (2017). "A unified ligand electronic parameter based on C NMR spectroscopy of N-heterocyclic carbene complexes". Dalton Transactions 46 (3): 614–627. doi:10.1039/C6DT04222H. PMID 27924321.
- ↑ Belcher R (1973). "Application of chelate Compounds in Analytical Chemistry". Pure and Applied Chemistry 34: 13–27. doi:10.1351/pac197334010013.
- ↑ Bellér, G. B.; Lente, G. B.; Fábián, I. N. (2010). "Central Role of Phenanthroline Mono-N-oxide in the Decomposition Reactions of Tris(1,10-phenanthroline)iron(II) and -iron(III) Complexes". Inorganic Chemistry 49 (9): 3968–3970. doi:10.1021/ic902554b. PMID 20415494.
- ↑ George B. Kauffman; Lloyd T. Takahashi (1966). Resolution of the tris-(1,10-Phenanthroline)Nickel(II) Ion. Inorganic Syntheses. 5. 227–232. doi:10.1002/9780470132395.ch60. ISBN 9780470132395.
- ↑ Armaroli N (2001). "Photoactive Mono- and Polynuclear Cu(I)-Phenanthrolines. A Viable Alternative to Ru(Ii)-Polypyridines?". Chemical Society Reviews 30 (2): 113–124. doi:10.1039/b000703j.
- ↑ 12.0 12.1 Pallenberg A. J.; Koenig K. S.; Barnhart D. M. (1995). "Synthesis and Characterization of Some Copper(I) Phenanthroline Complexes". Inorg. Chemistry 34 (11): 2833–2840. doi:10.1021/ic00115a009.
- ↑ Dwyer, F. P.; Gyarfas, Eleonora C.; Rogers, W. P.; Koch, Judith H. (1952). "Biological Activity of Complex Ions". Nature 170 (4318): 190–191. doi:10.1038/170190a0. PMID 12982853. Bibcode: 1952Natur.170..190D.
- ↑ Felber, Jean-Pierre; Coombs, Thomas L.; Vallee, Bert L. (1962). "The mechanism of inhibition of carboxypeptidase A by 1,10-phenanthroline". Biochemistry 1 (2): 231–238. doi:10.1021/bi00908a006. PMID 13892106.
- ↑ Salvesen, GS; Nagase, H (2001). "Inhibition of proteolytic enzymes". in Beynon, Rob. Proteolytic Enzymes: A Practical Approach. 1 (2nd ed.). Oxford University Press. pp. 105–130. ISBN 9780199636624.
- ↑ Preston, H. S.; Kennard, C. H. L. (1969). "Crystal Structure of di-mu-Chloro-sym-trans-Dichloro-Bis-(2,9-Dimethyl-1,10-Phenanthroline)dinickel(II)-2-Chloroform". J. Chem. Soc. A: 2682–2685. doi:10.1039/J19690002682.
- ↑ Leipoldt, J.G.; Lamprecht, G.J.; Steynberg, E.C. (1991). "Kinetics of the substitution of acetylacetone in acetylactonato-1,5-cyclooctadienerhodium(I) by derivatives of 1,10-phenantrholine and 2,2′-dipyridyl". Journal of Organometallic Chemistry 402 (2): 259–263. doi:10.1016/0022-328X(91)83069-G.
- ↑ Altman, Ryan A. (2008). "Encyclopedia of Reagents for Organic Synthesis". eEROS. doi:10.1002/047084289X.rn00918. ISBN 978-0471936237.
- ↑ Fagan, Paul J.; Nugent, William A. (1998). "1-Phenyl-2,3,4,5-Tetramethylphosphole". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV9P0653.; Collective Volume, 9, pp. 653
- ↑ Lin, Ho-Shen; Paquette, Leo A. (1994). "A Convenient Method for Determining the Concentration of Grignard Reagents". Synth. Commun. 24 (17): 2503–2506. doi:10.1080/00397919408010560.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.444. ISBN 9781498754293.

