Chemistry:Pinocembrin
From HandWiki
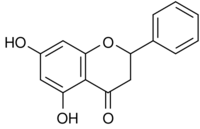
| |
| Names | |
|---|---|
| IUPAC name
5,7-Dihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one
| |
| Other names
Dihydrochrysin
Galangin flavanone 5,7-Dihydroxyflavanone 5,7-Dihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-phenyl- 5,7-Dihydroxy-2-phenyl-chroman-4-one | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H12O4 | |
| Molar mass | 256.257 g·mol−1 |
| Density | 1.386 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pinocembrin is a flavanone, a type of flavonoid. It is an antioxidant found in damiana,[1] honey, fingerroot,[2] and propolis.[3]
Pinocembrin can be converted biosynthetically to pinobanksin by hydroxylation adjacent to the ketone. Studies have shown that pinocembrin has potential as a drug to treat cerebral ischemia, intracerebral hemorrhage, neurodegenerative diseases, cardiovascular diseases and atherosclerosis as well as other diseases.[4][5]
See also
References
- ↑ Zhao, J; Dasmahapatra AK; Khan SI; Khan IA (Dec 2008). "Anti-aromatase activity of the constituents from damiana (Turnera diffusa)". J Ethnopharmacol 120 (3): 387–393. doi:10.1016/j.jep.2008.09.016. PMID 18948180.
- ↑ Punvittayagul, C; Wongpoomchai R; Taya S; Pompimon W. (January 2011). "Effect of pinocembrin isolated from Boesenbergia pandurata on xenobiotic-metabolizing enzymes in rat liver.". Drug Metabolism Letters 5 (1): 1–5. doi:10.2174/187231211794455226. PMID 20942797.
- ↑ Bosio K; Avanzini C; D’Avolio A; Ozino O; Savoia D (2000). "In vitro activity of propolis against Streptococcus pyogenes". Letters in Applied Microbiology 31 (2): 174–177. doi:10.1046/j.1365-2672.2000.00785.x. PMID 10972723.
- ↑ "The natural flavonoid pinocembrin: molecular targets and potential therapeutic applications". Mol Neurobiol 53 (3): 1794–801. April 2016. doi:10.1007/s12035-015-9125-2. PMID 25744566.
- ↑ "Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia". Brain Behav. Immun. 61: 326–339. March 2017. doi:10.1016/j.bbi.2016.12.012. PMID 28007523.
External links
 |
