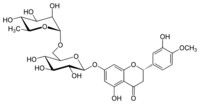
Chemistry:Hesperidin
| |

| |
| Names | |
|---|---|
| IUPAC name
(2S)-3′,5-Dihydroxy-4′-methoxy-7-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavan-4-one
| |
| Systematic IUPAC name
(22S,42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-13,25,43,44,45,73,74,75-Octahydroxy-14-methoxy-76-methyl-22,23-dihydro-24H-3,6-dioxa-2(2,7)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-24-one | |
| Other names
Hesperetin, 7-rutinoside,[1] Cirantin, hesperidoside|heperetin, 7-rhamnoglucoside, hesperitin, 7-O-rutinoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H34O15 | |
| Molar mass | 610.565 g·mol−1 |
| Density | 1.65 ± 0.1g/mL (predicted) |
| Melting point | 262 °C |
| Boiling point | 930.1 ± 65 °C (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist M. Lebreton from the white inner layer of citrus peels (mesocarp, albedo).[2][3]
Hesperidin is believed to play a role in plant defense.
Sources
Rutaceae
- 700–2,500 ppm in fruit of Citrus aurantium (bitter orange, petitgrain)[4]
- in orange juice (Citrus sinensis)
- in Zanthoxylum gilletii[5]
- in lemon[6]
- in lime[6]
- in leaves of Agathosma serratifolia
Lamiaceae
Peppermint contains hesperidin.[7]
Content in foods
Approximate hesperidin content per 100 ml or 100 g[8]
- 481 mg peppermint, dried
- 44 mg blood orange, pure juice
- 26 mg orange, pure juice
- 18 mg lemon, pure juice
- 14 mg lime, pure juice
- 1 mg grapefruit, pure juice
Metabolism
Hesperidin 6-O-α-L-rhamnosyl-β-D-glucosidase, an enzyme that uses hesperidin and water to produce hesperetin and rutinose, is found in the Ascomycetes species.[9]
Research
As a flavanone found in the rinds of citrus fruits (such as oranges or lemons), hesperidin is under preliminary research for its possible biological properties in vivo. One review did not find evidence that hesperidin affected blood lipid levels or hypertension.[10] Another review found that hesperidin may improve endothelial function in humans, but the overall results were inconclusive.[11]
Biosynthesis
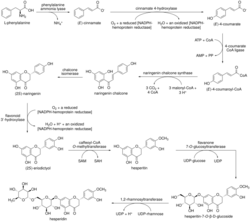
The biosynthesis of hesperidin stems from the phenylpropanoid pathway, in which the natural amino acid L-phenylalanine undergoes a deamination by phenylalanine ammonia lyase to afford (E)-cinnamate.[12] The resulting monocarboxylate undergoes an oxidation by cinnamate 4-hydroxylase to afford (E)-4-coumarate,[13] which is transformed into (E)-4-coumaroyl-CoA by 4-coumarate-CoA ligase.[14] (E)-4-coumaroyl-CoA is then subjected to the type III polyketide synthase naringenin chalcone synthase, undergoing successive condensation reactions and ultimately a ring-closing Claisen condensation to afford naringenin chalcone.[15] The corresponding chalcone undergoes an isomerization by chalcone isomerase to afford (2S)-naringenin,[16] which is oxidized to (2S)-eriodictyol by flavonoid 3′-hydroxylase.[17] After O-methylation by caffeoyl-CoA O-methyltransferase,[18] the hesperitin product undergoes a glycosylation by flavanone 7-O-glucosyltransferase to afford hesperitin-7-O-β-D-glucoside.[19] Finally, a rhamnosyl moiety is introduced to the monoglycosylated product by 1,2-rhamnosyltransferase, forming hesperidin.[20]
See also
- Diosmin
- List of phytochemicals in food
- List of MeSH codes (D03)
- List of food additives
References
- ↑ "Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed, Pluchea lanceolata (DC) C.B. Clarke (Asteraceae)". Journal of Chemical Ecology 17 (8): 1585–1591. August 1991. doi:10.1007/BF00984690. PMID 24257882.
- ↑ "Sur la matière cristalline des orangettes, et analyse de ces fruits non encore developpés, famille des Hesperidées". Journal de Pharmacie et de Sciences Accessories 14: 377. 1828. http://gallica.bnf.fr/ark:/12148/bpt6k214864s/f380.image. Retrieved 2016-10-30.
- ↑ "Metabocard for Hesperidin (HMDB03265)". Human Metabolome Database, The Metabolomics Innovation Centre, Genome Canada. 11 February 2016. http://www.hmdb.ca/metabolites/HMDB03265.
- ↑ "Citrus aurantium L.". Dr. Duke's Phytochemical and Ethnobotanical Databases. 6 Oct 2014. http://sun.ars-grin.gov:8080/npgspub/xsql/duke/plantdisp.xsql?taxon=276.
- ↑ "Antifeedant constituents from Fagara macrophylla". Fitoterapia 72 (5): 538–543. June 2001. doi:10.1016/S0367-326X(01)00265-9. PMID 11429249.
- ↑ 6.0 6.1 "Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature". Journal of Food Composition and Analysis 19 (Supplement): S74–S80. 2006. doi:10.1016/j.jfca.2005.12.009. http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Articles/jfca19_S74-S80.pdf. Retrieved 2014-01-24.
- ↑ "UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha × piperita L.)". Journal of Photochemistry and Photobiology B: Biology 100 (2): 67–75. August 2010. doi:10.1016/j.jphotobiol.2010.05.003. PMID 20627615.
- ↑ "Foods in which hesperidin is found". Phenol-Explorer database, version 3.6. http://phenol-explorer.eu/contents/polyphenol/207.
- ↑ "Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid beta-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria". Archives of Microbiology 192 (5): 383–393. May 2010. doi:10.1007/s00203-010-0567-7. PMID 20358178.
- ↑ "Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials". Phytotherapy Research 33 (3): 534–545. March 2019. doi:10.1002/ptr.6264. PMID 30632207.
- ↑ "Effects of hesperidin consumption on cardiovascular risk biomarkers: a systematic review of animal studies and human randomized clinical trials". Nutrition Reviews 77 (12): 845–864. December 2019. doi:10.1093/nutrit/nuz036. PMID 31271436.
- ↑ "The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana". Plant Molecular Biology 27 (2): 327–338. January 1995. doi:10.1007/BF00020187. PMID 7888622.
- ↑ "Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta". Plant Physiology 113 (3): 755–763. March 1997. doi:10.1104/pp.113.3.755. PMID 9085571.
- ↑ "Characterization in vitro and in vivo of the putative multigene 4-coumarate:CoA ligase network in Arabidopsis: syringyl lignin and sinapate/sinapyl alcohol derivative formation". Phytochemistry 66 (17): 2072–2091. September 2005. doi:10.1016/j.phytochem.2005.06.022. PMID 16099486.
- ↑ "Low Temperature Induces the Accumulation of Phenylalanine Ammonia-Lyase and Chalcone Synthase mRNAs of Arabidopsis thaliana in a Light-Dependent Manner". Plant Physiology 108 (1): 39–46. May 1995. doi:10.1104/pp.108.1.39. PMID 12228452.
- ↑ "Reaction mechanism of chalcone isomerase. pH dependence, diffusion control, and product binding differences". The Journal of Biological Chemistry 277 (2): 1361–1369. January 2002. doi:10.1074/jbc.m109224200. PMID 11698411.
- ↑ "Identification of the Arabidopsis thaliana flavonoid 3′-hydroxylase gene and functional expression of the encoded P450 enzyme". Biological Chemistry 381 (8): 749–753. August 2000. doi:10.1515/BC.2000.095. PMID 11030432.
- ↑ "Combinatorial biosynthesis of plant-specific coumarins in bacteria". Metabolic Engineering 18: 69–77. July 2013. doi:10.1016/j.ymben.2013.04.004. PMID 23644174.
- ↑ "Flavanone-7-O-glucosyltransferase activity from Petunia hybrida". Phytochemistry 52 (5): 793–798. November 1999. doi:10.1016/S0031-9422(99)00307-6. PMID 10626374.
- ↑ "UDP-rhamnose:flavanone-7-O-glucoside-2″-O-rhamnosyltransferase". The Journal of Biological Chemistry 266 (31): 20953–20959. November 1991. doi:10.1016/s0021-9258(18)54803-1. PMID 1939145.
External links
 |
