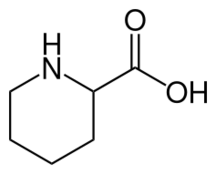
Chemistry:Pipecolic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidine-2-carboxylic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | C031345 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H11NO2 | |
| Molar mass | 129.15704 |
| Appearance | white or colorless solid |
| Melting point | 268 °C (514 °F; 541 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivative of piperidine and, as such, an amino acid, although not one encoded genetically. Like many other α-amino acids, pipecolic acid is chiral, although the S-stereoisomer is more common. It is a colorless solid.
Its biosynthesis starts from lysine.[1] CRYM, a taxon-specific protein that also binds thyroid hormones, is involved in the pipecolic acid pathway.
Medicine
It accumulates in pipecolic acidemia. Elevation of pipecolic acid can be associated with some forms of epilepsy, such as pyridoxine-dependent epilepsy.[2][3]
Occurrence and reactions
Like most amino acids, pipecolic acid is a chelating agent. One complex is Cu(HNC5H9CO2)2(H2O)2.[4]
Pipecolic acid was identified in the Murchison meteorite.[5] It also occurs in the leaves of the genus Myroxylon, a tree from South America.[6]
See also
- Bupivacaine
- Efrapeptin
References
- ↑ Gatto, Gregory J.; Boyne, Michael T.; Kelleher, Neil L.; Walsh, Christopher T. (2006). "Biosynthesis of Pipecolic Acid by RapL, a Lysine Cyclodeaminase Encoded in the Rapamycin Gene Cluster". Journal of the American Chemical Society 128 (11): 3838–3847. doi:10.1021/ja0587603. PMID 16536560.
- ↑ "Pipecolic acid as a diagnostic marker of pyridoxine-dependent epilepsy". Neuropediatrics 36 (3): 200–5. 2005. doi:10.1055/s-2005-865727. PMID 15944906.
- ↑ "Pyridoxine-Dependent Epilepsy and Antiquitin Deficiency Resulting in Neonatal-Onset Refractory Seizures". Brain Sciences 12 (1): 65. December 2021. doi:10.3390/brainsci12010065. PMID 35053812.
- ↑ Hockless, David C.R.; Mayadunne, Renuka C.; Wild, S.Bruce (1995). "Convenient resolution of (±)-piperidine-2-carboxylic acid ((±)-pipecolic acid) by separation of palladium(II) diastereomers containing orthometallated (S)-(−)-1-[1-(dimethylamino)ethyl]naphthalene". Tetrahedron: Asymmetry 6 (12): 3031–3037. doi:10.1016/0957-4166(95)00400-9.
- ↑ Kvenholden, Keith A.; Lawless, James G.; Ponnamperuma, Cyril (February 1971). "Nonprotein Amino Acids in the Murchison Meteorite". Proceedings of the National Academy of Sciences 68 (2): 486–490. doi:10.1073/pnas.68.2.486. PMID 16591908. Bibcode: 1971PNAS...68..486K.
- ↑ Kite, GC; Cardoso, D; Lewis, GP; Zartman, CE; de Queiroz, LP; Veitch, NC (2015). "Monomethyl ethers of 4,5-dihydroxypipecolic acid from Petaladenium urceoliferum: Enigmatic chemistry of an enigmatic legume". Phytochemistry 116: 198–202. doi:10.1016/j.phytochem.2015.02.026. PMID 25817832.
 |
