Chemistry:Polyetheramines
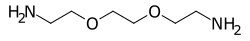
Polyetheramines are a group of chemicals that are aliphatic organic species based on both ether and amine groups. They are produced by reacting either ethylene oxide or propylene oxide with polyols and then aminating them. There are a number of commercially available molecules with different CAS numbers and molecular weights. They often come with a prefix of M, D or T for monofunctional, difunctional and trifunctional respectively. D-230 would mean difunctional with a molecular weight of 230.[1] A key use is for curing epoxy resins.
Use as epoxy resin curatives
One of the primary uses of polyether amines is as an epoxy curing agent.[2] They are commercially available as mono, di and tri-functional.[3] Difunctional versions tend to give flexibility and thus toughness to epoxy resin systems.[4][5][6] Studies have been done on the cured properties of epoxy systems using different functionality polyetheramines.[7] As epoxy curatives, they may then be further formulated into CASE applications: coatings, adhesives, sealants, and elastomers.
A key component of a Mannich base apart from formaldehyde and a phenolic species, is an amine. Polyetheramines can undergo the Mannich reaction and thus may be used to make Mannich bases.[8]
Use as a fuel additive
Sludge and other deposits build up in internal combustion engines especially gasoline powered versions. Fuel additives and detergents are thus often employed to help remove these or at least minimize them. Polyetheramines are one such additive.[9][10][11][12][13]
References
- ↑ "Epoxy formulations using polyetheramines". https://polymerinnovationblog.com/wp-content/uploads/2020/08/Huntsman-epoxy_formulations_using_jeffamine_polyetheramines.pdf.
- ↑ Zhao, Heng; Xu, Shuangshuang; Guo, Anru; Li, Jie; Liu, Dong (2021-08-01). "The Curing Kinetics Analysis of Four Epoxy Resins Using a Diamine Terminated Polyether as Curing Agent". Thermochimica Acta 702: 178987. doi:10.1016/j.tca.2021.178987. ISSN 0040-6031. https://www.sciencedirect.com/science/article/pii/S0040603121001283.
- ↑ McAninch, Ian M.; Palmese, Giuseppe R.; Lenhart, Joseph L.; La Scala, John J. (2013-11-05). "Characterization of epoxies cured with bimodal blends of polyetheramines" (in en). Journal of Applied Polymer Science 130 (3): 1621–1631. doi:10.1002/app.39322. ISSN 0021-8995. https://onlinelibrary.wiley.com/doi/10.1002/app.39322.
- ↑ Su, Shunsheng; Wang, Haiqing; Zhou, Chuanjian; Wang, Yanxiang; Liu, Jianjun (2018-09-01). "Study on epoxy resin with high elongation-at-break using polyamide and polyether amine as a two-component curing agent" (in en). E-Polymers 18 (5): 433–439. doi:10.1515/epoly-2017-0252. ISSN 1618-7229. https://www.degruyter.com/document/doi/10.1515/epoly-2017-0252/html.
- ↑ Abdollahi, H.; Salimi, A.; Barikani, M.; Samadi, A.; Hosseini Rad, S.; Zanjanijam, A. R. (2019-03-05). "Systematic investigation of mechanical properties and fracture toughness of epoxy networks: Role of the polyetheramine structural parameters" (in en). Journal of Applied Polymer Science 136 (9). doi:10.1002/app.47121. ISSN 0021-8995. https://onlinelibrary.wiley.com/doi/10.1002/app.47121.
- ↑ Jamshidi, Hajar; Akbari, Reza; Beheshty, Mohammad Hosain (2015-05-01). "Toughening of dicyandiamide-cured DGEBA-based epoxy resins using flexible diamine" (in en). Iranian Polymer Journal 24 (5): 399–410. doi:10.1007/s13726-015-0332-5. ISSN 1735-5265. https://doi.org/10.1007/s13726-015-0332-5.
- ↑ Wan, Jintao; Li, Cheng; Bu, Zhi-Yang; Xu, Cun-Jin; Li, Bo-Geng; Fan, Hong (2012-04-15). "A comparative study of epoxy resin cured with a linear diamine and a branched polyamine". Chemical Engineering Journal 188: 160–172. doi:10.1016/j.cej.2012.01.134. ISSN 1385-8947. https://www.sciencedirect.com/science/article/pii/S1385894712001763.
- ↑ Lin, J. J.; Speranza, G. P.; Waddill, H. G. (1997-12-19). "Synthesis and reactivity of Mannich-derived polyoxyethylene amines as epoxy curing agents" (in en). Journal of Applied Polymer Science 66 (12): 2339–2346. doi:10.1002/(SICI)1097-4628(19971219)66:12<2339::AID-APP15>3.0.CO;2-V. ISSN 0021-8995. https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-4628(19971219)66:123.0.CO;2-V.
- ↑ Wang, Wenying; Wang, Wei; Zhu, Zhongpeng; Hu, Xiaoming; Qiao, Fulin; Yang, Jing; Liu, Dan; Chen, Pu et al. (2023-04-15). "Quantitation of polyetheramines as the active components of detergent additives in gasoline by the ninhydrin reaction". Fuel 338: 127275. doi:10.1016/j.fuel.2022.127275. ISSN 0016-2361. https://www.sciencedirect.com/science/article/pii/S0016236122040996.
- ↑ Kuo, Chung-Hao; Smocha, Ruth; Loeper, Paul; Mukkada, Nicholas; Simpson Green, Felicia (2022-08-30). "Aftermarket Fuel Additives and their Effects on GDI Injector Performance and Particulate Emissions" (in en). SAE Technical Paper Series (400 Commonwealth Drive, Warrendale, PA, United States: SAE International) 1. doi:10.4271/2022-01-1074. https://doi.org/10.4271/2022-01-1074.
- ↑ Zerda, T. W; Yuan, X; Moore, S. M (2001-08-01). "Effects of fuel additives on the microstructure of combustion engine deposits". Carbon 39 (10): 1589–1597. doi:10.1016/S0008-6223(00)00287-6. ISSN 0008-6223. https://www.sciencedirect.com/science/article/pii/S0008622300002876.
- ↑ "A Brief Look at Polyetheramine - A Unique and Powerful Fuel Additive". https://www.irocoatingadditive.com/polyetheramine/a-brief-look-at-polyetheramine-a-unique-and-powerful-fuel-additive.html.
- ↑ Smocha, Ruth (2020-09-15) (in English). Sludge and Varnish Evaluation of Polyether Amine Gasoline Fuel Additives at "Complete Fuel System Cleaner" Aftermarket Fuel Additive Concentrations (Report). Warrendale, PA: SAE Technical Paper. https://www.sae.org/publications/technical-papers/content/2020-01-2100/.
 |
