Chemistry:Propargylamine
From HandWiki
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| C3H5N | |
| Molar mass | 55.080 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.867 g/cm3 |
| Boiling point | 83 °C (181 °F; 356 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
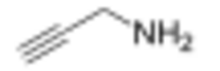
Propargylamine is an organic compound with the formula HC≡CCH2NH2. It is a colorless, odorless liquid that is used as a precursor to other compounds.[1] Propargyl amines are produced by reactions of amines with propargyl halides.
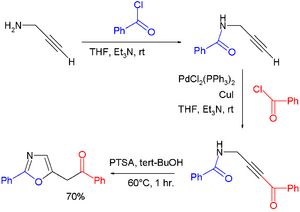
The behavior of propargyl amine is illustrated by its acylation benzoyl chloride to the amide. A Sonogashira coupling of the terminal alkyne end with another equivalent of benzoylchloride gives the dicarbonyl, a precursor to an oxazole.[2]
References
- ↑ Lauder, Kate; Toscani, Anita; Scalacci, Nicolò; Castagnolo, Daniele (2017). "Synthesis and Reactivity of Propargylamines in Organic Chemistry". Chemical Reviews 117 (24): 14091–14200. doi:10.1021/acs.chemrev.7b00343. PMID 29166000. https://kclpure.kcl.ac.uk/portal/en/publications/synthesis-and-reactivity-of-propargylamines-in-organic-chemistry(47919fe8-e7fc-4b3b-9296-e921fcf9673f).html.
- ↑ A new consecutive three-component oxazole synthesis by an amidation–coupling–cycloisomerization (ACCI) sequence Eugen Merkul and Thomas J. J. Müller Chem. Commun., 2006, 4817 - 4819, doi:10.1039/b610839c
 |