Chemistry:Pyridinium chloride
From HandWiki
Short description: Chemical compound
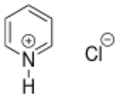 The chemical structure of pyridinium chloride
| |
| |
| Names | |
|---|---|
| IUPAC name
Pyridinium chloride
| |
| Other names
Pyridine hydrochloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6NCl | |
| Molar mass | 115.56 g/mol |
| Appearance | Hygroscopic white crystals |
| Density | 1.34 g/cm3 |
| Melting point | 144 °C (291 °F; 417 K) |
| Boiling point | Decomposes |
| 85 g / 100 mL | |
| Solubility | Soluble in chloroform, ethanol, insoluble in diethyl ether |
| Vapor pressure | 1 (0 °C) |
| Acidity (pKa) | 5 |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | [1] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Non-flammable | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1600 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pyridinium chloride is an organic chemical compound with a formula of C5H5NHCl.
Preparation
Pyridinium chloride can be produced by passing hydrogen chloride in pyridine dissolved in diethyl ether. The chemical formula is as follows:
- [math]\ce{ C5H5N + HCl -> C5H6N+Cl- v }[/math]
Acidity
Containing a pyridinium ion, pyridinium chloride has a pKa of approximately 5, slightly more acidic than that of typical amines. This is due to the hybridization of the nitrogen: the nitrogen is sp2 hybridized and more electronegative than those nitrogens in ammonium cations, which are sp3 hybridized. Hence they are stronger acids than amines and can be more easily deprotonated by bases.[3]
References
- ↑ "Pyridine hydrochloride(628-13-7) MSDS Melting Point Boiling Point Density Storage Transport". ChemicalBook. https://www.chemicalbook.com/ProductMSDSDetailCB0725473_EN.htm.
- ↑ Wilson, Michael W. (2001). "Encyclopedia of Reagents for Organic Synthesis" (in en). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289x.rp287m. ISBN 9780470842898.
- ↑ "Pyridine: synthesis and reactivity | BrainyResort" (in en-GB). 2016-11-08. https://www.brainyresort.com/en/pyridine-synthesis-and-reactivity/.
 |


