Chemistry:Saccharic acid
From HandWiki
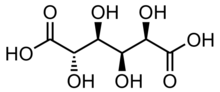
| |
| Names | |
|---|---|
| IUPAC name
D-glucaric acid
| |
| Other names
(2R,3S,4S,5S)-2,3,4,5-tetrahydroxyhexanedioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O8 | |
| Molar mass | 210.1388 |
| Melting point | 125-126 °C (decomposes) |
| Well soluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Saccharic acid, is a chemical compound with the formula C6H10O8. It is derived by oxidizing a sugar such as glucose with nitric acid.[1][2]
The salts of saccharic acid are called saccharates or glucarates.
See also
- Saccharide
- Disaccharides
- Monosaccharides
- Mucic acid
- Gluconic acid
- Isosaccharinic acid
References
- ↑ "Medical Definition of SACCHARIC ACID" (in en). https://www.merriam-webster.com/medical/saccharic+acid.
- ↑ "Saccharic acid" (in en). U.S. Department of Commerce. https://webbook.nist.gov/cgi/cbook.cgi?ID=87-73-0&Units=SI.
 |

