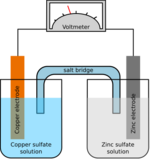
Chemistry:Salt bridge
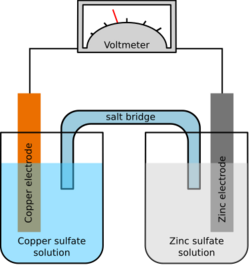
In electrochemistry, a salt bridge or ion bridge is an essential laboratory device discovered over 100 years ago. [1] It contains an electrolyte solution, typically an inert solution, used to connect the oxidation and reduction half-cells of a galvanic cell (voltaic cell), a type of electrochemical cell. [1][2] In short, it functions as a link connecting the anode and cathode half-cells within an electrochemical cell.[3] It also maintains electrical neutrality within the internal circuit and stabilizes the junction potential between the solutions in the half-cells.[4] Additionally, it serves to minimize cross-contamination between the two half cells. [1][5]
A salt bridge typically consists of tubes filled with an electrolyte solution. These tubes often have diaphragms such as glass frits at their ends to help contain the solution within the tubes and prevent excessive mixing with the surrounding environment.[3] When setting up a salt bridge between different solvents of half-cells, it is crucial to ensure that the electrolyte used in the bridge is soluble in both solutions and does not interact with any species present in either solutions. [3]
There are several types of salt bridges: glass tube bridges (traditional KCl-type salt bridge and ionic liquid salt bridge), filter paper bridges, porous frit salt bridges, fumed-silica, and agar gel salt bridges.
Glass tube bridges (KCl-type and ionic liquid salt bridge)
Glass tube salt bridges commonly consist of U-shaped Vycor tubes filled with a relatively inert electrolyte.[6] The electrolyte solution usually comprises a combination of cations, such as ammonium and potassium, and anions, including chloride and nitrate, which have similar mobility.[1][3] The combination is chosen which does not react with any of the chemicals used in the cell.
KCl-type salt bridges
Traditionally, concentrated aqueous potassium chloride (KCl) solution has been used for decades to neutralize the liquid-junction potential.[1] When comparing other salt solutions such as potassium bromide and potassium iodide to potassium chloride, potassium chloride is the most efficient in nullifying the junction potential.[1] Yet, the effectiveness of this salt bridge decreases as the ionic strength of the sample solution increases.[1]
Ionic liquid salt bridges
Due to the numerous drawbacks of KCl-type salt bridges, ionic liquid salt bridges (ILSB) have been utilized to address the potentiometry issues arising from KCl-type salt bridges in electrochemical cells.[1][4] ILSBs demonstrate efficient performance in aqueous solutions of hydrophilic electrolytes. This is because ionic liquids do not mix with water (they are immiscible), rendering them suitable as salt bridges for aqueous solutions.[1] Additionally, they are chemically inert and highly stable in water.[1]

To set up a glass tube salt bridge, a U-shaped Vycor tube is fashioned to contain a suitable electrolyte solution. [3] Normally, glass frits, a porous material, cover the ends of the tube or the electrolyte is often gelified with agar-agar to help prevent the intermixing of fluids that might otherwise occur.[3]
The conductivity of a glass tube bridge primarily depends on the concentration of the electrolyte solution. At concentrations below saturation, an increase in concentration enhances conductivity. However, beyond-saturation electrolyte content and a narrow tube diameter may both reduce conductivity.[4]
Filter paper bridges
Porous paper such as filter paper may be used as a salt bridge if soaked in an appropriate electrolyte such as the electrolytes used in glass tube bridges. No gelification agent is required as the filter paper provides a solid medium for conduction. [7]
The conductivity of this kind of salt bridge depends on a number of factors: the concentration of the electrolyte solution, the texture of the paper, and the absorbing ability of the paper. Generally, smoother texture and higher absorbency equate to higher conductivity. [7]
To set up this type of salt bridge, laboratory filter paper can be used and rolled to form a shape that connects the two half-cells, typically rolled into a cylindrical shape.[7] The rolled filter paper is then soaked in an appropriate inert salt solution.[7] A straw can be used to shape the rolled filter paper into a U-shaped tube, providing mechanical strength to the soaked filter paper.[7][8] This filter paper can now be used to act as a salt bridge and connect the two half-cells.[7]
While filter paper salt bridges are inexpensive and easily accessible, one disadvantage of not using a straw to provide mechanical strength is that a new rolled and soaked filter paper must be used for each experiment.[7] Additionally, filter paper has limited longevity and poses a high risk of contamination.[3]
Charcoal salt bridges
A recent development is the charcoal salt bridge. It is considered an excellent option for a porous junction for the reference electrode in an alkaline solution.[9]

A porous junction serves as a salt bridge between the two half-cells of reference and electrolyte solutions.[9] Other materials used for porous junctions, such as glass, Teflon, and agar gel, have their own benefits but also some significant drawbacks such as high cost and high risk of contamination.[9][3]
Therefore, the advantages of using charcoal as frits include its low cost and easy accessibility, as charcoal can be sourced from porous carbon materials.[9] Despite being fragile, charcoal facilitates efficient ion transfer due to its highly porous structure.[9]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 Kakiuchi, Takashi (2011-07-01). "Salt bridge in electroanalytical chemistry: past, present, and future" (in en). Journal of Solid State Electrochemistry 15 (7): 1661–1671. doi:10.1007/s10008-011-1373-0. ISSN 1433-0768. https://doi.org/10.1007/s10008-011-1373-0.
- ↑ "5. Electrochemical Cells" (in en). 2017-05-18. https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/In_Class_Activities/Electrochemical_Methods_of_Analysis/02_Text/5._Electrochemical_Cells.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Doménech-Carbó, Antonio (2013-11-01). "György Inzelt, Andrzej Lewenstam, and Fritz Scholz (eds.), Handbook of Reference Electrodes" (in en). Journal of Solid State Electrochemistry 17 (11): 2967–2968. doi:10.1007/s10008-013-2160-x. ISSN 1433-0768. https://doi.org/10.1007/s10008-013-2160-x.
- ↑ 4.0 4.1 4.2 Kakiuchi, Takashi (2014-08-01). "Ionic liquid salt bridge — Current stage and perspectives: A mini review". Electrochemistry Communications 45: 37–39. doi:10.1016/j.elecom.2014.05.016. ISSN 1388-2481. https://www.sciencedirect.com/science/article/pii/S1388248114001489.
- ↑ Anderson, Evan L.; Troudt, Blair K.; Bühlmann, Philippe (2020). "Critical Comparison of Reference Electrodes with Salt Bridges Contained in Nanoporous Glass with 5, 20, 50, and 100 nm Diameter Pores". Analytical Sciences 36 (2): 187–191. doi:10.2116/analsci.19P235. PMID 31495816. https://www.jstage.jst.go.jp/article/analsci/36/2/36_19P235/_article/-char/ja/.
- ↑ Clem, Ray G.; Jakob, Fredi.; Anderberg, Dane. (1971-02-01). "Fumed silica salt-bridges" (in en). Analytical Chemistry 43 (2): 292–293. doi:10.1021/ac60297a014. ISSN 0003-2700. https://pubs.acs.org/doi/abs/10.1021/ac60297a014.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Malike Devi, G.; Padmavathi, D. A.; Sanjeev, R.; Jagannadham, V. (2016). "Filter Paper Salt Bridge in Potentiometric Titrations: An Undergraduate Laboratory Chemical Education Article". World Journal of Chemical Education 4 (6): 117–123. http://pubs.sciepub.com/wjce/4/6/1.
- ↑ "CEJ Vol. 8, No. 2 (Serial No. 15). Reg. No. 8-11". http://www.edu.utsunomiya-u.ac.jp/chem/v8n2/kumar/DKumar.html.
- ↑ 9.0 9.1 9.2 9.3 9.4 Lee, Jun-Seob (2020-02-15). "Use of a charcoal salt bridge to a reference electrode in an alkaline solution". Journal of Electroanalytical Chemistry 859. doi:10.1016/j.jelechem.2020.113872. ISSN 1572-6657. https://www.sciencedirect.com/science/article/pii/S1572665720300552.
 |