Chemistry:Vanadium redox battery
| Specific energy | 10–20 Wh/kg (36–72 J/g) |
|---|---|
| Energy density | 15–25 Wh/L (54–65 kJ/L) |
| Time durability | 20–30 years |
| Cycle durability | >12,000–14,000 cycles[1] |
| Nominal cell voltage | 1.15–1.55 V |
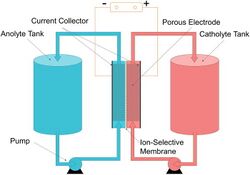

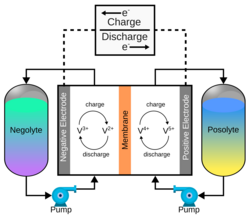
The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery. It employs vanadium ions as charge carriers.[3] The battery uses vanadium's ability to exist in a solution in four different oxidation states to make a battery with a single electroactive element instead of two.[4] For several reasons, including their relative bulkiness, vanadium batteries are typically used for grid energy storage, i.e., attached to power plants/electrical grids.[5]
Numerous companies and organizations are involved in funding and developing vanadium redox batteries.
History
Pissoort mentioned the possibility of VRFBs in the 1930s.[6] NASA researchers and Pellegri and Spaziante followed suit in the 1970s,[7] but neither was successful. Maria Skyllas-Kazacos presented the first successful demonstration of an All-Vanadium Redox Flow Battery employing dissolved vanadium in a solution of sulfuric acid in the 1980s.[8][9][10] Her design used sulfuric acid electrolytes, and was patented by the University of New South Wales in Australia in 1986.[11]
One of the important breakthroughs achieved by Skyllas-Kazacos and coworkers was the development of a number of processes to produce vanadium electrolytes of over 1.5 M concentration using the lower cost, but insoluble vanadium pentoxide as starting material. These processes involved chemical and electrochemical dissolution and were patented by the University of NSW in 1989. During the 1990s the UNSW group conducted extensive research on membrane selection,[12][13] graphite felt activation,[14][15] conducting plastic bipolar electrode fabrication,[16] electrolyte characterisation and optimisation as well as modelling and simulation. Several 1-5 kW VFB prototype batteries were assembled and field tested in a Solar House in Thailand and in an electric golf cart at UNSW.[17]
The UNSW All-Vanadium Redox Flow Battery patents and technology were licensed to Mitsubishi Chemical Corporation and Kashima-Kita Electric Power Corporation in the mid-1990s and subsequently acquired by Sumitomo Electric Industries where extensive field testing was conducted in a wide range of applications in the late 1990s and early 2000s.[18]
In order to extend the operating temperature range of the battery and prevent precipitation of vanadium in the electrolyte at temperatures above 40oC in the case of V(V), or below 10oC in case of the negative half-cell solution, Skyllas-Kazacos and coworkers tested hundreds of organic and inorganic additives as potential precipitation inhibitors. They discovered that inorganic phosphate and ammonium compounds were effective in inhibiting precipitation of 2 M vanadium solutions in both the negative and positive half-cell at temperatures of 5 and 45 °C respectively and ammonium phosphate was selected as the most effective stabilising agent. Ammonium and phosphate additives were used to prepare and test a 3 M vanadium electrolyte in a flow cell with excellent results.[18]

Advantages and disadvantages
Advantages
VRFBs' main advantages over other types of battery:[20]
- no limit on energy capacity
- can remain discharged indefinitely without damage
- mixing electrolytes causes no permanent damage
- single charge state across the electrolytes avoids capacity degradation
- safe, non-flammable aqueous electrolyte
- no noise or emissions
- battery modules can be added to meet demand
- wide operating temperature range including passive cooling[21][22]
- long charge/discharge cycle lives: 15,000-20,000 cycles and 10–20 years.
- low levelized cost: (a few tens of cents), approaching the 2016 $0.05 target stated by the United States Department of Energy and the European Commission Strategic Energy Technology Plan €0.05 target.[23]
Disadvantages
VRFBs' main disadvantages compared to other types of battery:[20]
- high and volatile prices of vanadium minerals (i.e. the cost of VRFB energy)
- relatively poor round trip efficiency (compared to lithium-ion batteries)
- heavy weight of aqueous electrolyte
- relatively poor energy-to-volume ratio compared to standard storage batteries
- having moving parts in the pumps that produce the flow of electrolyte solution
- toxicity of vanadium (V) compounds.
Materials
A vanadium redox battery consists of an assembly of power cells in which two electrolytes are separated by a proton exchange membrane. The electrodes in a VRB cell are carbon based. The most common types are carbon felt, carbon paper, carbon cloth, graphite felt, and carbon nanotubes.[24][25][26] The carbon-based electrode exhibits limited catalytic activity when interacting with vanadium species. To enhance its catalytic performance, several approaches have been employed, including thermal treatment, acid treatment, electrochemical modification, and the incorporation of catalysts. [27][28]
Both electrolytes are vanadium-based. The electrolyte in the positive half-cells contains VO2+ and VO2+ ions, while the electrolyte in the negative half-cells consists of V3+ and V2+ ions. The electrolytes can be prepared by several processes, including electrolytically dissolving vanadium pentoxide (V2O5) in sulfuric acid (H2SO4).[29] The solution is strongly acidic in use.
The most common membrane material is perfluorinated sulfonic acid (PFSA or Nafion). However, vanadium ions can penetrate a PFSA membrane and destabilize the cell.[30] A 2021 study found that penetration is reduced with hybrid sheets made by growing tungsten trioxide nanoparticles on the surface of single-layered graphene oxide sheets. These hybrid sheets are then embedded into a sandwich structured PFSA membrane reinforced with polytetrafluoroethylene (Teflon). The nanoparticles also promote proton transport, offering high Coulombic efficiency and energy efficiency of more than 98.1 percent and 88.9 percent, respectively.[31]
Operation
The reaction uses the half-reactions:[32]
Other useful properties of vanadium flow batteries are their fast response to changing loads and their overload capacities. They can achieve a response time of under half a millisecond for a 100% load change, and allow overloads of as much as 400% for 10 seconds. Response time is limited mostly by the electrical equipment. Unless specifically designed for colder or warmer climates, most sulfuric acid-based vanadium batteries work between about 10 and 40 °C. Below that temperature range, the ion-infused sulfuric acid crystallizes.[35] Round trip efficiency in practical applications is around 70–80%.[36]
Proposed improvements
The original VRFB design by Skyllas-Kazacos employed sulfate (added as vanadium sulfate(s) and sulfuric acid) as the only anion in VRFB solutions, which limited the maximum vanadium concentration to 1.7 M of vanadium ions.[37] In the 1990s, Skyllas-Kazacos discovered the use of ammonium phosphate and other inorganic compounds as precipitation inhibitors to stabilise 2 M vanadium solutions over a temperature range of 5 to 45 oC and a Stabilising Agent patent was filed by UNSW in 1993. This discovery was largely overlooked however and in around 2010 a team from Pacific Northwest National Laboratory proposed a mixed sulfate-chloride electrolyte, that allowed for the use in VRFBs solutions with the vanadium concentration of 2.5 M over a whole temperature range between −20 and +50 °C.[38][39] Based on the standard equilibrium potential of the V5+/V4+ couple it is expected to oxidize chloride, and for this reason chloride solutions were avoided in earlier VRFB studies. The surprising oxidative stability (albeit only at the state of charge below ca. 80%) of V5+ solutions in the presence of chloride was explained on the basis of activity coefficients.[40] Many researchers explain the increased stability of V(V) at elevated temperatures by the higher proton concentration in the mixed acid electrolyte that shifts the thermal precipitation equilibrium of V(V) away from V2O5. Nevertheless, because of a high vapor pressure of HCl solutions and the possibility of chlorine generation during charging, such mixed electrolytes have not been widely adopted.[41]
Another variation is the use of vanadium bromide salts. Since the redox potential of Br2/2Br- couple is more negative than that of V5+/V4+, the positive electrode operates via the bromine process.[42] However, due to problems with volatility and corrosivity of Br2, they did not gain much popularity (see zinc-bromine battery for a similar problem). A vanadium/cerium flow battery has also been proposed .[43]
Specific energy and energy density
VRBs achieve a specific energy of about 20 Wh/kg (72 kJ/kg) of electrolyte. Precipitation inhibitors can increase the density to about 35 Wh/kg (126 kJ/kg), with higher densities possible by controlling the electrolyte temperature. The specific energy is low compared to other rechargeable battery types (e.g., lead–acid, 30–40 Wh/kg (108–144 kJ/kg); and lithium ion, 80–200 Wh/kg (288–720 kJ/kg)).[citation needed]
Applications
VRFBs' large potential capacity may be best-suited to buffer the irregular output of utility-scale wind and solar systems.[20]
Their reduced self-discharge makes them potentially appropriate in applications that require long-term energy storage with little maintenance—as in military equipment, such as the sensor components of the GATOR mine system.[44][20]
They feature rapid response times well suited to uninterruptible power supply (UPS) applications, where they can replace lead–acid batteries or diesel generators. Fast response time is also beneficial for frequency regulation. These capabilities make VRFBs an effective "all-in-one" solution for microgrids, frequency regulation and load shifting.[20]
Largest vanadium grid batteries
| Name | Commissioning date | Energy (MWh) | Power (MW) | Duration (hours) | Country |
|---|---|---|---|---|---|
| Minami Hayakita Substation[45][46] | December 2015 | 60 | 15 | 4 | Japan |
| Pfinztal, Baden-Württemberg[47][48][49] | September 2019 | 20 | 2 | 10 | Germany |
| Woniushi, Liaoning[50][51] | 10 | 5 | 2 | China | |
| Tomamae Wind Farm[52] | 2005 | 6 | 4 | 1:30 | Japan |
| Zhangbei Project[53] | 2016 | 8 | 2 | 4 | China |
| SnoPUD MESA 2 Project[54][55] | March 2017 | 8 | 2 | 4 | USA |
| San Miguel Substation[56] | 2017 | 8 | 2 | 4 | USA |
| Pullman Washington[57] | April 2015 | 4 | 1 | 4 | USA |
| Dalian Battery[58] | October 2022 | 400 (800) | 100 (200) | 4 | China |
Companies funding or developing vanadium redox batteries
Companies funding or developing vanadium redox batteries include Sumitomo Electric Industries,[59] CellCube (Enerox),[60] UniEnergy Technologies,[61] StorEn Technologies[62][63] in Australia, Largo Energy[64] and Ashlawn Energy[65] in the United States; H2 in Gyeryong-si, South Korea;[66] Renewable Energy Dynamics Technology,[67] Invinity Energy Systems[68] in the United Kingdom, VoltStorage[69] and Schmalz[70][71] in Europe; Prudent Energy[72] in China; Australian Vanadium, CellCube and North Harbour Clean Energy[73][74] in Australia; Yadlamalka Energy Trust and Invinity Energy Systems[75][76] in Australia; EverFlow Energy JV SABIC SCHMID Group in Saudi Arabia[77] and Bushveld Minerals in South Africa.[78]
See also
Citations
- ↑ Electricity Storage and Renewables: Costs and Markets to 2030. IRENA (2017), Electricity Storage and Renewables: Costs and Markets to 2030, International Renewable Energy Agency, Abu Dhabi.
- ↑ Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena 35 (4): 040801. doi:10.1116/1.4983210. ISSN 2166-2746. Bibcode: 2017JVSTB..35d0801Q.
- ↑ Laurence Knight (14 June 2014). "Vanadium: The metal that may soon be powering your neighbourhood". BBC. https://www.bbc.com/news/magazine-27829874.
- ↑ Alotto, P.; Guarnieri, M.; Moro, F. (2014). "Redox Flow Batteries for the storage of renewable energy: a review". Renewable & Sustainable Energy Reviews 29: 325–335. doi:10.1016/j.rser.2013.08.001.
- ↑ James Purtill (2 February 2023). "Vanadium redox flow batteries can provide cheap, large-scale grid energy storage. Here's how they work.". Australian Broadcasting Corporation. https://www.abc.net.au/news/science/2023-02-02/vanadium-redox-flow-battery-and-future-of-grid-energy-storage/101911604.
- ↑ P. A. Pissoort, in FR Patent 754065 (1933)
- ↑ A. Pelligri and P. M. Spaziante, in GB Patent 2030349 (1978), to Oronzio de Nori Impianti Elettrochimici S.p.A.
- ↑ Rychik, M.; Skyllas-Kazacos, M. (January 1988). "Characteristics of a new all-vanadium redox flow battery". Journal of Power Sources 22 (1): 59–67. doi:10.1016/0378-7753(88)80005-3. Bibcode: 1988JPS....22...59R.
- ↑ "Discovery and invention: How the vanadium flow battery story began". 18 October 2021. https://www.energy-storage.news/discovery-and-invention-how-the-vanadium-flow-battery-story-began/.
- ↑ "Vanadium Redox Battery | UNSW Research". https://research.unsw.edu.au/projects/vanadium-redox-battery.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedSkyllas - ↑ Chieng, S.C.; Kazacos, M.; Skyllas-Kazacos, M. (1992). "Preparation and evaluation of composite membrane for vanadium redox battery applications". Journal of Power Sources 39 (1): 11–19. doi:10.1016/0378-7753(92)85002-R. Bibcode: 1992JPS....39...11C.
- ↑ Chieng, S.C.; Kazacos, M.; Skyllas-Kazacos, M. (16 December 1992). "Modification of Daramic, microporous separator, for redox flow battery applications". Journal of Membrane Science 75 (1–2): 81–91. doi:10.1016/0376-7388(92)80008-8.
- ↑ Sun, B.; Skyllas-Kazacos, M. (June 1992). "Modification of graphite electrode materials for vanadium redox flow battery application—I. Thermal treatment". Electrochimica Acta 37 (7): 1253–1260. doi:10.1016/0013-4686(92)85064-R.
- ↑ Sun, Bianting; Skyllas-Kazacos, Maria (October 1992). "Chemical modification of graphite electrode materials for vanadium redox flow battery application—part II. Acid treatments". Electrochimica Acta 37 (13): 2459–2465. doi:10.1016/0013-4686(92)87084-D.
- ↑ Zhong, S.; Kazacos, M.; Burford, R.P.; Skyllas-Kazacos, M. (October 1991). "Fabrication and activation studies of conducting plastic composite electrodes for redox cells". Journal of Power Sources 36 (1): 29–43. doi:10.1016/0378-7753(91)80042-V. Bibcode: 1991JPS....36...29Z.
- ↑ Tang, Ao; McCann, John; Bao, Jie; Skyllas-Kazacos, Maria (November 2013). "Investigation of the effect of shunt current on battery efficiency and stack temperature in vanadium redox flow battery". Journal of Power Sources 242: 349–356. doi:10.1016/j.jpowsour.2013.05.079. Bibcode: 2013JPS...242..349T.
- ↑ 18.0 18.1 Skyllas-Kazacos, Maria (1 July 2022). "Review—Highlights of UNSW All-Vanadium Redox Battery Development: 1983 to Present". Journal of the Electrochemical Society 169 (7): 070513. doi:10.1149/1945-7111/ac7bab. Bibcode: 2022JElS..169g0513S.
- ↑ Tolmachev, Yuriy V. (1 March 2023). "Review—Flow Batteries from 1879 to 2022 and Beyond". Journal of the Electrochemical Society 170 (3): 030505. doi:10.1149/1945-7111/acb8de. Bibcode: 2023JElS..170c0505T.
- ↑ 20.0 20.1 20.2 20.3 20.4 Ragsdale, Rose (May 2020). "Vanadium fuels growing demand for VRFBs" (in en). https://www.metaltechnews.com/story/2020/05/27/tech-metals/vanadium-fuels-growing-demand-for-vrfbs/240.html.
- ↑ "Vanadium Redox Flow Batteries". Pacific Northwest National Laboratory. October 2012. http://energy.gov/sites/prod/files/VRB.pdf.
- ↑ Miller, Kelsey. UniEnergy Technologies Goes from Molecules to Megawatts , Clean Tech Alliance, 7 July 2014. Accessed 21 January 2016.
- ↑ Spagnuolo, G.; Petrone, G.; Mattavelli, P.; Guarnieri, M. (2016). "Vanadium Redox Flow Batteries: Potentials and Challenges of an Emerging Storage Technology". IEEE Industrial Electronics Magazine 10 (4): 20–31. doi:10.1109/MIE.2016.2611760.
- ↑ Mustafa, Ibrahim; Lopez, Ivan; Younes, Hammad; Susantyoko, Rahmat Agung; Al-Rub, Rashid Abu; Almheiri, Saif (March 2017). "Fabrication of Freestanding Sheets of Multiwalled Carbon Nanotubes (Buckypapers) for Vanadium Redox Flow Batteries and Effects of Fabrication Variables on Electrochemical Performance". Electrochimica Acta 230: 222–235. doi:10.1016/j.electacta.2017.01.186. ISSN 0013-4686.
- ↑ Mustafa, Ibrahim; Bamgbopa, Musbaudeen O.; Alraeesi, Eman; Shao-Horn, Yang; Sun, Hong; Almheiri, Saif (2017-01-01). "Insights on the Electrochemical Activity of Porous Carbonaceous Electrodes in Non-Aqueous Vanadium Redox Flow Batteries". Journal of the Electrochemical Society 164 (14): A3673–A3683. doi:10.1149/2.0621714jes. ISSN 0013-4651.
- ↑ Mustafa, Ibrahim; Al Shehhi, Asma; Al Hammadi, Ayoob; Susantyoko, Rahmat; Palmisano, Giovanni; Almheiri, Saif (May 2018). "Effects of carbonaceous impurities on the electrochemical activity of multiwalled carbon nanotube electrodes for vanadium redox flow batteries". Carbon 131: 47–59. doi:10.1016/j.carbon.2018.01.069. ISSN 0008-6223.
- ↑ He, Zhangxing; Lv, Yanrong; Zhang, Tianao; Zhu, Ye; Dai, Lei; Yao, Shuo; Zhu, Wenjie; Wang, Ling (January 2022). "Electrode materials for vanadium redox flow batteries: Intrinsic treatment and introducing catalyst". Chemical Engineering Journal 427: 131680. doi:10.1016/j.cej.2021.131680.
- ↑ Bourke, Andrea; Oboroceanu, Daniela; Quill, Nathan; Lenihan, Catherine; Safi, Maria Alhajji; Miller, Mallory A.; Savinell, Robert F.; Wainright, Jesse S. et al. (1 March 2023). "Review—Electrode Kinetics and Electrolyte Stability in Vanadium Flow Batteries". Journal of the Electrochemical Society 170 (3): 030504. doi:10.1149/1945-7111/acbc99. Bibcode: 2023JElS..170c0504B.
- ↑ Guo, Yun; Huang, Jie; Feng, Jun-Kai (February 2023). "Research progress in preparation of electrolyte for all-vanadium redox flow battery". Journal of Industrial and Engineering Chemistry 118: 33–43. doi:10.1016/j.jiec.2022.11.037.
- ↑ Zhang, Yue; Zhang, Denghua; Luan, Chao; Zhang, Yifan; Yu, Wenjie; Liu, Jianguo; Yan, Chuanwei (24 February 2023). "An Economical Composite Membrane with High Ion Selectivity for Vanadium Flow Batteries". Membranes 13 (3): 272. doi:10.3390/membranes13030272. PMID 36984659.
- ↑ Lavars, Nick (2021-11-12). "Hybrid membrane edges flow batteries toward grid-scale energy storage" (in en-US). https://newatlas.com/energy/hybrid-membrane-battery-grid-scale-energy-storage/.
- ↑ Jin, Jutao; Fu, Xiaogang; Liu, Qiao; Liu, Yanru; Wei, Zhiyang; Niu, Kexing; Zhang, Junyan (25 June 2013). "Identifying the Active Site in Nitrogen-Doped Graphene for the VO 2+ /VO 2 + Redox Reaction". ACS Nano 7 (6): 4764–4773. doi:10.1021/nn3046709. PMID 23647240.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ Atkins, Peter (2010). Inorganic Chemistry (5th ed.). W. H. Freeman. pp. 153. ISBN 978-1-42-921820-7.
- ↑ DOE/Pacific Northwest National Laboratory (17 March 2011). "Electric Grid Reliability: Increasing Energy Storage in Vanadium Redox Batteries by 70 Percent". Science Daily. https://www.sciencedaily.com/releases/2011/03/110317141418.htm.
- ↑ Revankar, Shripad T. (2019). "Chapter 6. Chemical Energy Storage". Storage and Hybridization of Nuclear Energy – Techno-economic Integration of Renewable and Nuclear Energy. London: Academic Press. pp. 177–227. doi:10.1016/B978-0-12-813975-2.00006-5. ISBN 9780128139752.
- ↑ M. Skyllas-Kazacos, M. Rychcik and G. Robins Robert, "All vanadium redox battery." 1986AU-0055562 1986-04-02. M. Skyllas-Kazacos, "All-vanadium redox battery and additives." 1988WO-AU00472 1988-12-091989AU-0028153 1989-12-09. M. Skyllas-Kazacos, M. Kazacos and C. Mcdermott Rodney John, "Vanadium charging cell and vanadium dual battery system." 1989AU-0028152 1989-12-09. M. Kazacos and S. Kazacos Maria, "High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions." 1996AT-0911853T 1996-05-031996AU-0054914 1996-05-031996US-08945869 1996-05-031996WO-AU00268 1996-05-031996NZ-0306364 1996-05-031996ES-0911853T 1996-05-031996EP-0911853 1996-05-031996DE-6030298 1996-05-031996CA-2220075 1996-05-031998HK-0110321 1998-08-312002US-10226751 2002-08-22
- ↑ Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G. et al. (2011). "A stable vanadium redox-flow battery with high energy density for large-scale energy storage". Advanced Energy Materials 1 (3): 394–400. doi:10.1002/aenm.201100008.
- ↑ Yang, Y.; Zhang, Y.; Tang, L.; Liu, T.; Huang, J.; Peng, S.; Yang, X. (September 2019). "Investigations on physicochemical properties and electrochemical performance of sulfate-chloride mixed acid electrolyte for vanadium redox flow battery". Journal of Power Sources 434: Article 226719. doi:10.1016/j.jpowsour.2019.226719. Bibcode: 2019JPS...43426719Y.
- ↑ Roznyatovskaya, N.; Noack, J.; Mild, H.; Fühl, M.; Fischer, P.; Pinkwart, K.; Tübke, J.; Skyllas-Kazacos, M. (2019). "Vanadium electrolyte for all-vanadium redox-flow batteries: the effect of the counter ion". Batteries 5 (1): 13. doi:10.3390/batteries5010013.
- ↑ Yuriy V Tolmachev. Review—Flow Batteries From 1879 To 2022 And Beyond. https://iopscience.iop.org/article/10.1149/1945-7111/acb8de/meta
- ↑ Vafiadis, Helen; Skyllas-Kazacos, Maria (2006). "Evaluation of membranes for the novel vanadium bromine redox flow cell". Journal of Membrane Science 279 (1–2): 394–402. doi:10.1016/j.memsci.2005.12.028.
- ↑ Sankarasubramanian, Shrihari; Zhang, Yunzhu; Ramani, Vijay (2019). "Methanesulfonic acid-based electrode-decoupled vanadium–cerium redox flow battery exhibits significantly improved capacity and cycle life" (in en). Sustainable Energy & Fuels 3 (9): 2417–2425. doi:10.1039/C9SE00286C. ISSN 2398-4902. http://xlink.rsc.org/?DOI=C9SE00286C.
- ↑ Allbright, Greg, et al. A Comparison of Lead Acid to Lithium-ion in Stationary Storage Applications All Cell, March 2012
- ↑ Stone, Mike (3 February 2016). "A Look at the Biggest Energy Storage Projects Built Around the World in the Last Year". https://www.greentechmedia.com/articles/read/a-look-at-the-biggest-energy-storage-projects-built-around-the-world-in-the.
- ↑ "DOE Global Energy Storage Database". https://www.energystorageexchange.org/projects/2043.
- ↑ "Redox-Flow-Batterien". http://www.carmen-ev.de/sonne-wind-co/stromspeicher/batterien/315-akkutypen/650-redox-flow-batterien.
- ↑ Armin Herberger (19 January 2021). "Hybridspeichersystem in Wohnquartier – KIT plant in Bruchsal Weltpremiere mit Strom-Wärme-Kopplung". Badische Neueste Nachrichten Kraichgau. https://bnn.de/kraichgau/bruchsal/kit-plant-in-bruchsal-weltpremiere-mit-strom-waerme-kopplung.
- ↑ "Großprojekt "RedoxWind"". Fraunhofer-Institut für Chemische Technologie. https://www.ict.fraunhofer.de/de/komp/ae/RFBWind.html.
- ↑ "Energy Storage in China". ees-magazine.com. http://www.ees-magazine.com/energy-storage-in-china/?upm_export=print.
- ↑ Zonghao, L. I. U.; Huamin, Zhang; Sujun, G. a. O.; Xiangkun, M. A.; Yufeng, L. I. U.; 刘宗浩, 张华民. "The world's largest all-vanadium redox flow battery energy storage system for a wind farm, 风场配套用全球最大全钒液流电池储能系统". 储能科学与技术 3 (1): 71–77. doi:10.3969/j.issn.2095-4239.2014.01.010. http://energystorage-journal.com:81/EN/10.3969/j.issn.2095-4239.2014.01.010.
- ↑ "DOE Global Energy Storage Database". https://www.energystorageexchange.org/projects/599.
- ↑ "DOE Global Energy Storage Database". https://www.energystorageexchange.org/projects/2026.
- ↑ "UET and Snohomish County PUD Dedicate the World's Largest Capacity Containerized Flow Battery". Energy Storage News. 29 March 2017. http://energystorage.org/news/esa-news/uet-and-snohomish-county-pud-dedicate-worlds-largest-capacity-containerized-flow.
- ↑ "PUD invests $11.2 million in energy-storing units". Everett Herald. 2 November 2016. http://www.heraldnet.com/news/pud-invests-in-11-2-million-in-energy-storing-units/.
- ↑ "SDG&E and Sumitomo unveil largest vanadium redox flow battery in the US". Energy Storage News. 17 March 2017. https://www.energy-storage.news/news/sdge-and-sumitomo-unveil-largest-vanadium-redox-flow-battery-in-the-us.
- ↑ Wesoff, Eric, St. John, Jeff. Largest Capacity Flow Battery in North America and EU is Online, Greentech Media, June 2015. Accessed 21 January 2016.
- ↑ "World's largest flow battery connected to the grid in China" (in en-US). 2022-10-03. https://newatlas.com/energy/worlds-largest-flow-battery-grid-china/.
- ↑ "Redox Flow Battery" (in en-US). https://sumitomoelectric.com/products/redox/features.
- ↑ "CellCube – the versatile energy storage system of the future" (in en-US). https://www.cellcube.com/.
- ↑ Steve Wilhelm (3 July 2014). "Liquid battery the size of a truck, will give utilities a charge". Puget Sound Business Journal. http://www.bizjournals.com/seattle/news/2014/07/03/liquid-battery-the-size-of-a-truck-will-give.html?page=all.
- ↑ Entrepreneur, Office of the Queensland Chief (2021-02-03). "How Queensland can supercharge the future of batteries" (in en). https://www.chiefentrepreneur.qld.gov.au/news/2020/how-queensland-can-supercharge-the-future-of-batteries.
- ↑ "StorEn Tech Provides First of Its Kind Vanadium Flow Battery To Australia" (in en-US). 2020-12-19. https://cleantechnica.com/2020/12/19/storen-tech-provides-first-of-its-kind-vanadium-flow-battery-to-australia/.
- ↑ "Vanadium producer Largo prepares 1.4GWh of flow battery stack manufacturing capacity". 6 May 2021. https://www.energy-storage.news/news/vanadium-producer-largo-prepares-1.4gwh-of-flow-battery-stack-manufacturing.
- ↑ BILL HAGSTRAND (23 August 2013). "Vanadium redox: powering up local communities". Crain's Cleveland Business. http://www.crainscleveland.com/article/20130823/BLOGS05/308239999/vanadium-redox-powering-up-local-communities.
- ↑ Andy Colthorpe (14 November 2022). "South Korean flow battery maker H2 building 330MWh factory". Energy Storage News. https://www.energy-storage.news/south-korean-flow-battery-maker-h2-building-330mwh-factory/.
- ↑ "US clean-tech investments leap to US$1.1bn. Where's Ireland at?". Silicon Republic. 11 April 2011. http://www.siliconrepublic.com/clean-tech/item/24346-us-clean-tech-investments-l.
- ↑ "'UK's first' grid-scale battery storage system comes online in Oxford". 24 June 2021. https://www.euractiv.com/section/electricity/news/uks-first-grid-scale-battery-storage-system-comes-online-in-oxford/.
- ↑ "Voltstorage develops a safe and ecological storage solution". 16 January 2018. http://www.pveurope.eu/Products/Storage/Batteries/Voltstorage-develops-a-safe-and-ecological-storage-solution.
- ↑ "Lösungen für Energiespeichersysteme: Schmalz baut weiteres Geschäftsfeld auf". 2016-06-16. https://www.windkraft-journal.de/2016/06/16/loesungen-fuer-energiespeichersysteme-schmalz-baut-weiteres-geschaeftsfeld-auf/86788.
- ↑ "Stacks of Schmalz". 2023-05-28. https://www.schmalz-energy.com/en.
- ↑ Jeff St. John (2 March 2010). "Made in China: Prudent Energy Lands $22M For Flow Batteries". GigaOm. http://gigaom.com/cleantech/made-in-china-prudent-energy-lands-22m-for-flow-batteries/.
- ↑ "Australian Vanadium Ltd ships first vanadium flow battery from Austria". Proactive Investors. 13 July 2016. http://www.proactiveinvestors.com.au/companies/news/132721/australian-vanadium-ltd-ships-first-vanadium-flow-battery-from-austria-69782.html.
- ↑ "Vanadium flow battery partners sign agreement to develop gigafactory in Australia". VSUN Energy. 24 November 2022. http://vsunenergy.com.au/news/vanadium-flow-battery-partners-sign-agreement-to-develop-gigafactory-in-australia/.
- ↑ "Renewable technology solutions to enable a sustainable energy future". Yadlamalka Energy. 2023. http://www.yadlamalkaenergy.com/.
- ↑ Gabriella Marchant (4 January 2021). "Australian Renewable Energy Agency backs vanadium flow battery project in outback SA". Australian Broadcasting Corporation. https://www.abc.net.au/news/2020-12-14/australian-renewable-energy-agency-vanadium-flow-battery-sa/12979270.
- ↑ "3GWh flow battery manufacturing facility to be constructed in Saudi Arabia". 16 May 2020. https://constructionreviewonline.com/news/3gwh-flow-battery-manufacturing-facility-to-be-constructed-in-saudi-arabia/.
- ↑ "Vanadium producer Bushveld Minerals begins building flow battery electrolyte plant in South Africa". 15 June 2021. https://www.energy-storage.news/news/vanadium-producer-bushveld-minerals-begins-building-flow-battery-electrolyt.
General and cited references
- Presentation paper from the IEEE summer 2001 conference
- UNSW Site on Vanadium batteries
- Report by World Energy
- World Map Of Global Vanadium Deposits Vanadium geology is fairly unusual compared to a base metals ore body.
- "Improved Redox Flow Batteries For Electric Cars". ScienceDaily/Fraunhofer-Gesellschaft. 13 October 2009. https://www.sciencedaily.com/releases/2009/10/091012135506.htm.
External links
- The U.S. made a breakthrough battery discovery – then gave the technology to China The U.S. made a breakthrough battery discovery – then gave the technology to China
- VRFB developments at UNSW
- The Need for Vanadium Redox Energy Storage in Wind Turbine Generators—Net electricity generation from all forms of renewable energies in America increased by over 15% between 2005 and 2009
- redT and Avalon have merged as Invinity Energy Systems, a global leader in Vanadium Flow Batteries
 |