Chemistry:Seamanite
| Seamanite | |
|---|---|
 Seamanite crystals on a rock sample (5 x 4 x 3 cm) | |
| General | |
| Category | Borate minerals |
| Formula (repeating unit) | Mn3[B(OH)4](PO4)(OH)2[1] |
| Strunz classification | 6.AC.65[2] |
| Dana classification | 43.4.5.1[1] |
| Crystal system | Orthorhombic |
| Crystal class | Dipyramidal (mmm) H-M symbol: (2/m 2/m 2/m)[3] |
| Space group | Pbnm |
| Unit cell | a = 7.811 Å, b = 15.114 Å c = 6.691 Å, Z = 4 |
| Identification | |
| Formula mass | 372.64 g/mol[2] |
| Color | yellow, yellow-brown, pink[1] |
| Crystal habit | acicular[2] |
| Cleavage | distinct on {001}[3] |
| Fracture | brittle[2] |
| Tenacity | brittle[3] |
| Mohs scale hardness | 4[1] |
| |re|er}} | vitreous[2] |
| Streak | white[2] |
| Diaphaneity | transparent[3] |
| Specific gravity | 3.08[1] |
| Density | 3.08–3.128 g/cm3[3] |
| Refractive index | nα = 1.640, nβ = 1.663, nγ = 1.665[4] |
| Birefringence | δ = 0.025[1] |
| 2V angle | ≈40°[4] |
| Dispersion | weak[1] |
| Ultraviolet fluorescence | none[2] |
| Solubility | in cold, dilute acids[1] |
| References | [3] |
Seamanite, named for discoverer Arthur E. Seaman, is a rare manganese boron phosphate mineral with formula Mn3[B(OH)4](PO4)(OH)2. The yellow to pink mineral occurs as small, needle-shaped crystals. It was first discovered in 1917 from a mine in Iron County, Michigan, United States and identified in 1930. As of 2012[update], seamanite is known from four sites in Michigan and South Australia.
History
In 1917, Arthur E. Seaman collected a mineral sample from the Chicagon Mine in Iron County, Michigan.[lower-alpha 1] He correctly believed it to be a new mineral species based on a qualitative analysis of its composition by F. B. Wilson. World War I delayed further study of the mineral until 1929. A study in 1930 proved it to be a new mineral and named it seamanite in honor of Seaman. They cited his career as a professor of geology and mineralogy and his contributions to the field as reasons for the naming.[6]
The original analysis of the mineral in 1930 suggested seamanite to be a hydrated salt.[7] However, in 1971, the mineral was determined to be the coordination compound Mn3[B(OH)4](PO4)(OH)2.[8]
Description
Seamanite is a transparent, yellow to pink mineral that occurs as needle-shaped crystals.[2] Seamanite is a brittle mineral with a mohs hardness of 4.[1] It is found in the crevices of fractured siliceous rock.[6] The type occurrence was found in association with small crystals of calcite, thin coatings of manganese oxide,[6] and fibrous sussexite.[9] Seamanite has also been found with shigaite.[10]
Distribution
As of 2012[update], seamanite is known from four locations: the Cambria-Jackson Mine in Marquette County, Michigan, the Chicagon Mine and the Bengal Mine in Iron County, Michigan, and the Iron Monarch open cut in the Eyre Peninsula, South Australia.[1]
The type material is stored at Michigan Technological University in Houghton, Michigan, and at the National Museum of Natural History in Washington, D.C. as sample 96282.[3]
Crystallography
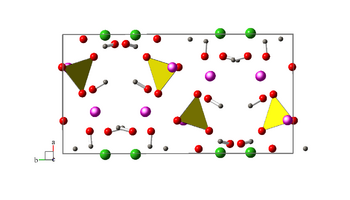
gray:H red:O green:B violet:Mn center of yellow tetrahedrons:P
Seamanite is formed of acicular crystals elongated along [001] and showing the faces {110} and {111} up to one centimeter. It has an orthorhombic crystal system and the Pbnm space group. The parameters of its unit cell are: a=7.811 Å, b=15.114 Å, c=6.691 Å, Z=4 units per unit cell.[3]
Notes
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 "Seamanite". Mindat. http://www.mindat.org/min-3599.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Seamanite Mineral Data". Webmineral. http://www.webmineral.com/data/Seamanite.shtml.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Seamanite". Handbook of Mineralogy. Mineral Data Publishing. http://www.handbookofmineralogy.org/pdfs/seamanite.pdf.
- ↑ 4.0 4.1 Kraus, p. 222
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine 85 (3): 291–320. doi:10.1180/mgm.2021.43. Bibcode: 2021MinM...85..291W.
- ↑ 6.0 6.1 6.2 Kraus, p. 220.
- ↑ Kraus, p. 223–5
- ↑ Moore, p. 1527.
- ↑ Slawson, p. 575
- ↑ "Seamanite – Photo Gallery". Mindat. http://www.mindat.org/gallery.php?cform_is_valid=1&min=3599&cf_pager_page=1.
Bibliography
- Kraus, E.H.; Seaman, W.A.; Slawson, C.B. (June 1930). "Seamanite, a new manganese phospho-borate from Iron County, Michigan". American Mineralogist (Mineralogical Society of America) 15 (6): 220–225. http://www.minsocam.org/ammin/AM15/AM15_220.pdf. Retrieved April 13, 2012.
- Moore, Paul B.; Ghose, Subrata (September–October 1971). "A Novel Face-Sharing Octahedral Trimer in the Crystal Structure of Seamanite". American Mineralogist (Mineralogical Society of America) 56 (9 & 10): 1527–1538. http://www.minsocam.org/ammin/am56/am56_1527.pdf. Retrieved April 13, 2012.
- Palache, P.; Berman H.; Frondel, C. (1960). "Dana's System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, Etc. (Seventh Edition)" John Wiley and Sons, Inc., New York, pp. 388–389.
- Slawson, Chester B. (December 1934). "Sussexite from Iron County, Michigan". American Mineralogist (Mineralogical Society of America) 19 (12): 575–578. http://www.minsocam.org/ammin/AM19/AM19_575.pdf. Retrieved April 13, 2012.
Further reading
- Huminicki, Danielle M.C.; Hawthorne, Frank C. (2002). "Hydrogen Bonding in the Crystal Structure of Seamanite". The Canadian Mineralogist 40 (3): 923–928. doi:10.2113/gscanmin.40.3.923. http://rruff.geo.arizona.edu/doclib/cm/vol40/CM40_923.pdf. Retrieved April 14, 2012.
- McConnell, Duncan; Pondrom, Walter L. Jr. (July 1941). "X-ray Crystallography of Seamanite". American Mineralogist (Mineralogical Society of America) 26 (7): 446–447. http://www.minsocam.org/ammin/AM26/AM26_446.pdf. Retrieved April 13, 2012.
External links
 |
