Chemistry:Sodium cobaltinitrite
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium hexanitritocobaltate(III)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Na 3[Co(NO 2) 6] | |
| Molar mass | 403.933 g·mol−1 |
| Appearance | Yellow crystals |
| Density | 2.565 g/cm3 |
| Hazards | |
| Safety data sheet | JT Baker MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
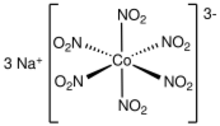
Sodium cobaltinitrite is inorganic compound with the formula Na
3[Co(NO
2)
6]. The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO
2)
6]3−. It was a reagent for the qualitative test for potassium and ammonium ions.[1]
Synthesis and reactions
The compound is prepared by oxidation of cobalt(II) salts in the presence of sodium nitrite:[2]
- 4 [Co(H
2O)
6](NO
3)
2 + O
2 + 24 NaNO
2 → 4 Na
3[Co(NO
2)
6] + 8 NaNO
3 + 4 NaOH + 22 H
2O
Application for analysis of potassium
Although the sodium cobaltinitrite is soluble in water, it forms the basis of a quantitative determination of potassium, thallium, and ammonium ions. Under the recommended reaction conditions the insoluble double salt, K
2Na[Co(NO
2)
6] · H2O is precipitated and weighed.[3] In geochemical analysis, sodium cobaltinitrite is used to distinguish alkali feldspars from plagioclase feldspars in thin section.[4]
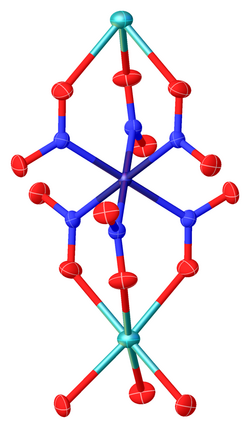
3Co(NO
2)
6 lattice, highlighting the CoN
6 and NaO
6 coordination spheres.[5]
See also
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Glemser, O. (1963). "Sodium Hexanitritocobaltate(III)". in Brauer, G.. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York, NY: Academic Press. p. 1541.
- ↑ Vogel, A. I. (1951). Quantitative Inorganic Analysis (2nd ed.). Longmans Green and Co..
- ↑ Bailey, E. H.; Stevens, R. E. (1960). "Selective staining of K-feldspar and plagioclase on rock slabs and thin sections". American Mineralogist 45: 1020–1025.
- ↑ Brian N. Figgis, Alexandre N. Soboleva (2001). "Na3Co(NO2)6 at 293 and 10 K". Acta Crystallographica Section C 57 (Pt 8): 885–886. doi:10.1107/S0108270101007995. PMID 11498599.

![K 2Na[Co(NO 2) 6] · H2O](/wiki/images/thumb/a/ab/Impure_dipotassium_sodium_cobaltinitrite_%28K2Na%28Co%28NO2%296%29%29_monohydrate.jpg/180px-Impure_dipotassium_sodium_cobaltinitrite_%28K2Na%28Co%28NO2%296%29%29_monohydrate.jpg)
