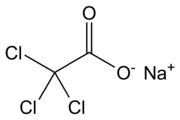
Chemistry:Sodium trichloroacetate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium trichloroacetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C2Cl3NaO2 | |
| Molar mass | 185.36 g/mol |
| Appearance | White powder |
| Density | ~1.5 g/mL−1 |
| Melting point | 200 °C (392 °F; 473 K) |
| Boiling point | Decomposes |
| 55 g / 100 ml | |
| Solubility | Soluble in methanol and ethanol, slightly soluble in acetone, not soluble in ethers and hydrocarbons |
| Acidity (pKa) | 0.7 (conjugate acid) |
| Hazards | |
| Main hazards | Corrosive |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H335, H410 | |
| P261, P271, P273, P304+340, P312, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Non-flammable | |
| Related compounds | |
Other anions
|
Sodium trifluoroacetate |
Other cations
|
Trichloroacetic acid |
Related compounds
|
Sodium chloroacetate Sodium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium trichloroacetate is a chemical compound with a formula of CCl3CO2Na. It is used to increase sensitivity and precision during transcript mapping.[1] It was previously used as an herbicide starting in the 1950s but regulators removed it from the market in the late 1980s and early 1990s.[2][3][4][5]
Preparation
Sodium trichloroacetate is made by reaction trichloroacetic acid with sodium hydroxide:
Reactions
Basicity
Sodium trichloroacetate is a weaker base than sodium acetate because of the electron-withdrawing nature of the trichloromethyl group. Sodium trifluoroacetate is likewise a weaker base. However, it can easily be protonated in the presence of suitably strong acids:
Trichloromethyl-anion precursor
This reagent is useful for introducing the trichloromethyl group into other molecules. Decarboxylation produces the trichloromethyl anion, which is a sufficiently strong nucleophile to attack various carbonyl functional groups, such as aldehydes, carboxylic acid anhydrides,[6] ketones (making a precursor for the Jocic–Reeve reaction), and acyl halides.
See also
References
- ↑ Murray, M. G. (1986). "Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping". Analytical Biochemistry 158 (1): 165–170. doi:10.1016/0003-2697(86)90605-6. ISSN 0003-2697. PMID 2432801.
- ↑ TCA-sodium in the Pesticide Properties DataBase (PPDB), accessed June 20, 2014
- ↑ G. S. Rai and C. L. Hamner Persistence of Sodium Trichloroacetate in Different Soil Types Weeds 2(4) Oct. 1953: 271-279
- ↑ OECD Trichloroacetic Acid CAS N°: 76-03-9 Accessed June 20, 2014
- ↑ EPA December 1991. trichloroacetic acid (TCA) EPA Cancellation 12/91 Accessed June 20, 2014
- ↑ Winston, Anthony; Bederka, John P. M.; Isner, William G.; Juliano, Peter C.; Sharp, John C. (1965). "Trichloromethylation of Anhydrides. Ring—Chain Tautomerism". J. Org. Chem. 30 (8): 2784–2787. doi:10.1021/jo01019a068.
 |

