Chemistry:Streptolidine
From HandWiki
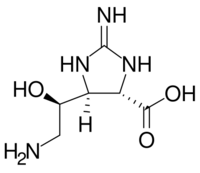
| |
| Names | |
|---|---|
| IUPAC name
(4S)-2-amino-5-[(1R)-2-amino-1-hydroxyethyl]-4,5-dihydro-1H-imidazole-4-carboxylic acid
| |
| Other names
Roseonine, AC1MIYK9
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H12N4O3 | |
| Molar mass | 188.187 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Streptolidine is an amino acid isolated from the hydrolyzate of the Streptomyces antibiotics streptothricin and streptolin.[1] Its structure was first elucidated by chemical degradation[2] and later by x-ray crystallography.[3]
Synthesis
Syntheses have been accomplished from D-ribose[4] and D-xylose.[5]
References
- ↑ Carter, H. E.; Sweeley, C. C.; Daniels, E. E.; McNary, J. E.; Schaffner, C. P.; West, C. A.; Van Tamelen, E. E.; Dyer, J. R. et al. (20 October 1961). "Streptothricin and Streptolin: The Structure of Streptolidine (Roseonine)". Journal of the American Chemical Society 83 (20): 4296–4297. doi:10.1021/ja01481a052.
- ↑ Hanessian, Stephen (1983). Total Synthesis of Natural Products: The 'Chiron' Approach. Pergamon press. pp. 173. ISBN 978-0-08-029247-2.
- ↑ Bycroft, B. W.; King, T. J. (1 January 1972). "Crystal structure of streptolidine, a guanidine-containing amino-acid". Journal of the Chemical Society, Chemical Communications (11): 652. doi:10.1039/C39720000652.
- ↑ Kusumoto, Shoichi, Shinichi Tsuji, and Tetsuo Shiba (1974). "Synthesis of streptolidine (roseonine, geamine)". Bulletin of the Chemical Society of Japan 47 (11): 2690–2695. doi:10.1246/bcsj.47.2690.
- ↑ Kusumoto, Shoichi (1976). "Synthesis of streptolidine from D-xylose". Bulletin of the Chemical Society of Japan 49 (12): 3611–3614. doi:10.1246/bcsj.49.3611.
 |

