Chemistry:Superelectrophilic anion
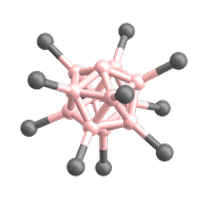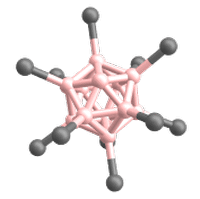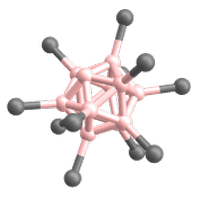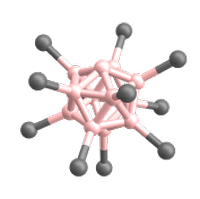
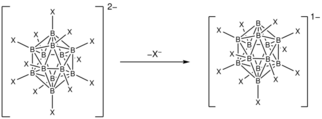
Superelectrophilic anions are a class of molecular ions that exhibit highly electrophilic reaction behavior despite their overall negative charge.[1][2] Thus, they are even able to bind the unreactive noble gases or molecular nitrogen at room temperature.[1][2][3] The only representatives known so far are the fragment ions of the type [B12X11]– derived from the closo-dodecaborate dianions [B12X12]2–. X represents a substituent connected to a boron atom (cf. Fig. 1). For this reason, the following article deals exclusively with superelectrophilic anions of this type.
Overview
Anions are negatively charged ions and therefore usually exhibit nucleophilic reaction behavior. However, it has been shown that there are anions which behave in a strongly electrophilic manner despite their negative charge. This means that they form bonds with reaction partners in chemical reactions by accepting electron density from them. Their affinity for electrons is so great that they are even able to bind very unreactive small molecules such as nitrogen (N2) or noble gas atoms at room temperature.[1][2][3] For this reason, they are called "superelectrophilic anions". Furthermore, these superelectrophilic anions allow the direct reaction with small alkanes such as methane.[4] Reactions with other anions are also possible and lead to the formation of highly charged ions.[5]
The only superelectrophilic anions known so far are the fragment ions with the molecular formula [B12X11]–, which can be generated from the closo-dodecaborate dianions [B12X12]2–. The X represents a substituent connected to a boron atom, e.g. Cl or Br.
Due to their high reactivity, these fragment ions can so far only be generated in the evacuated gas phase of a mass spectrometer. Therefore, reactions of this class of compounds have been studied mainly in the gas phase.[1][2][3][6] In the condensed phase, reaction products of the superelectrophilic anions were synthesized in small amounts using the ion soft-landing method.[4][5]
Potential applications of this research include the preparation of exotic compounds (e.g., noble gas compounds) of interest for fundamental chemical research. If syntheses with superelectrophilic anions would become possible on a larger scale, they might be used for applications, for example the development of cancer drugs for the Boron Neutron Capture Therapy (BNCT).[7]
Origin of the concept
Originally, the term superelectrophilic was used exclusively for dications.
It was introduced by George A. Olah when he discovered that certain electrophilic monocations can be activated in the condensed phase by the presence of particularly strong acids - the so-called superacids.[8] This makes these cations significantly more reactive than they are under normal conditions. He attributed this increased reactivity to the formation of doubly positively charged dications, which he termed "superelectrophilic."[9]
Later, the term superelectrophilic was also frequently applied in gas phase studies to highly reactive dications that can bind noble gases at room temperature. Noble gases are generally considered to be particularly inert - in order to form a stable bond with a noble gas atom, a pair of electrons must be abstracted from it. Only the strongest electrophiles are able to do this, and these are usually dications or, more rarely, monocations,[10][11] since high electrophilicity is accompanied by a substantial lack of electrons. In contrast, it is impossible for nucleophiles, which provide electrons for bond formation, to bind a noble gas strongly, because noble gases have negative electron affinities. Only weak interactions (ion- induced dipole and dispersion) are usually present, and do not result in a stable bond at room temperature.[8]
Since anions are negatively charged and formally have an electron excess, they generally exhibit nucleophilic reaction behavior and should therefore not be able to form stable bonds to noble gas atoms. However, in direct contrast to this intuitive concept, it was shown in 2017 that the negatively charged gas-phase fragment anion [B12Cl11]– can bind the noble gases krypton and xenon at room temperature and thus, must be strongly electrophilic.[2] Furthermore, the electrophilicity of this fragment anion could even be increased by exchanging the chlorine atoms (Cl) with cyano groups (CN).[1] The resulting [B12(CN)11]– anion also spontaneously binds the particularly unreactive noble gas argon at room temperature and neon up to a maximum temperature of 50 Kelvin.[6] Thus, it is the most electrophilic anion known to date.[1][6]
Even though anions cannot formally fulfill the concept of superelectrophilic published by Olah (which only refers to cations), these particular anions exhibit reactivity that strongly resembles that of superelectrophilic dications in the gas phase. Thus, the term superelectrophilic anions was used.[1]
Precursors
The superelectrophilic anions [B12X11]– can be generated from the very stable closo-dodecaborate dianions [B12X12]2– by fragmentation in the gas phase.
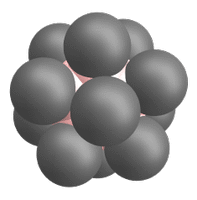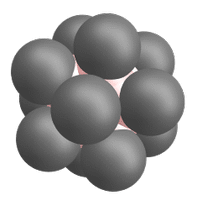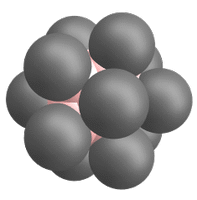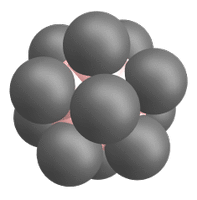
The closo-dodecaborate dianions, which serve as precursors, consist of twelve boron atoms arranged in a highly symmetrical, cage-like closo structure. All twelve boron atoms carry a substituent (e.g. a halogen atom), which together form the outer shell of the ion (see Fig. 3).
Due to their three-dimensional σ-aromatic structure, closo-dodecaborate dianions are remarkably stable compounds and can be used to stabilize reactive cations (cf. weakly coordinating anions).[12]
[B12X12]2– ions with the halogens (F, Cl, Br, I) or cyano groups (CN) as substituents (X) have been used as precursors for superelectrophilic anions so far.[1][2][3]
Generation
To generate the superelectrophilic anions from the closo-dodecaborate dianions [B12X12]2–, fragmentation is carried out in the mass spectrometer using collision-induced dissociation (CID). In this process, a substituent (X–) is cleaved from the dianion precursor. As a result, only eleven of the twelve boron atoms carry a substituent. One boron atom, on the other hand, is vacant and possesses a free binding site (cf. Figures 1 and 4).[1][2]
Depending on the nature of the substituents, this fragmentation can either occur directly by CID of the precursor [B12X12]2–, or by fragmentation of a so-called anionic ion pair M+[B12X12]2–, which loses the neutral particle MX using CID. The cation M+ can be, for example, an H+ ion or an alkali cation. Direct fragmentation of the precursor is possible for the chlorine, bromine and iodine variants (X = Cl, Br, I),[13] while generation of the anionic ion pair is required for the precursor variants with fluorine and cyano groups as substituents (X = F, CN).[1][13]
Characteristics
The vacant bonding site on one of the boron atoms created during fragmentation carries a substantial partial positive charge and is therefore strongly electrophilic, although the overall charge of the ion is negative (cf. Fig. 4). At this vacant boron atom, the lowest unoccupied molecular orbital (LUMO) with negative orbital energy is located and the delocalized σ-electron system is interrupted, as shown by Electron Localization Index (ELI) studies.[2]
The stiff and very stable dodecaborate framework prevents possible intramolecular rearrangement reactions, so that the highly reactive electrophilic center remains intact and chemically accessible.[1][2]
Reactivity and bond formation
Reactions with noble gases
The superelectrophilic anions exhibit special properties that together are very unusual and as a result allow the formation of stable noble gas bonds. These include the strong electrophilicity and structural stability of the anions, which have already been discussed in the Characteristics section. In addition, three further aspects were discussed which may be favourable for bond formation to noble gases:
Favored formation and stabilization of the collision complex
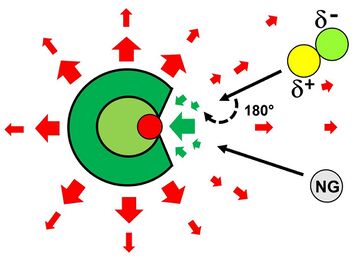
The strongly electrophilic (positive) binding site within the overall anionic framework generates an unusual electric field near the reactive boron atom. Polar nucleophiles approaching the anion in the preferred orientation must change their orientation to allow the nucleophilic site to react with the anion. This could result in a significant centrifugal barrier and reduce the number of reactive collisions. This effect has no influence on nonpolar molecules such as noble gases (see Fig. 5). The large molecular framework of these polyatomic cage anions allows the collision energy to be redistributed by the collision of the reactants over the many, especially low-energy, vibrational degrees of freedom, so that the collision complex is long-lived enough to be stabilized by collision cooling.[1]
Substitution protection
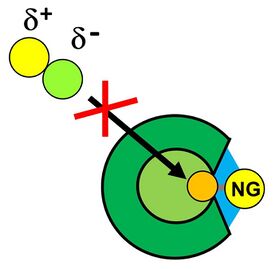
After the noble gas has been bound to the boron atom, its substitution by a nucleophile in a typical SN2 reaction is prevented by the cage structure of the borate, since the noble gas-boron bond is shielded sterically (cf. Fig. 6).[1]
Favorable electrostatics and dispersion forces
In addition to the coordinative noble gas-boron bond, dispersion interactions and electrostatics contribute to the stabilization of the noble gas complex. The electrophilic bonding site is located within a crater defined by five partially negatively charged substituents X, which provide a large interaction region for the formation of attractive London-dispersion forces. The bound noble gas atom, on the other hand, provides electron density to the neighboring boron atom and thus becomes weakly positive, which could play a role in an attractive and stabilizing electrostatic interaction with the surrounding substituents (see Fig. 6).[1]
For the reactions of the individual fragment ion variants with the various noble gases, see also section Variants.
Reactions with diatomic molecules: CO, N2
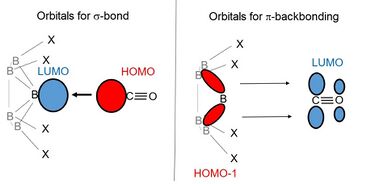
The binding of the diatomic molecules CO and N2 to superelectrophilic anions was studied using mass spectrometry, infrared photodissociation (IRPD) spectroscopy, and theoretical calculations. CO binds to the vacant boron atom of the electrophilic anion via its nucleophilic carbon. However, in contrast to the reaction with noble gases, the binding does not occur only through a σ-bonding of the nucleophile's CO and N2. The electrophilic anion pushes electron density into the antibonding π-orbitals of N2 and CO via π-backbonding, which further strengthens the bond to the electrophilic anion.[3]
For the reactions of the individual fragment ion variants with CO and N2, see also section Variants.
Reactions with saturated hydrocarbons
The high reactivity of the [B12X11]– anions allows not only reactions with noble gases, but also direct functionalization of saturated hydrocarbons.
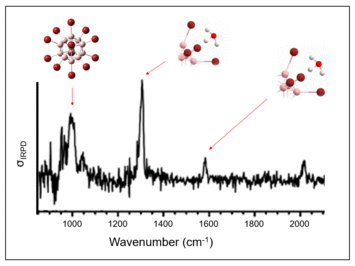
To elucidate the reaction mechanism, detailed investigations were carried out on the reactivity of [B12Br11]– and [B12Cl11]– with methane in the gas phase. The molecular structures were investigated with the aid of IR spectroscopy. It was shown that the electrophilic boron atom reacts with the less polar C–H bond. The carbon represents the partially negative (nucleophilic) part of the bond. A heterolytic bond cleavage of the C–H bond occurs, which formally leads to a carbanion [CH3]– and a proton H+. The carbanion binds to the electrophilic boron atom of [B12X11]–, forming the dianion [B12X11CH3]2–, while the H+ ion remains electrostatically bound to this dianion in the gas phase. The overall singly charged ion [B12X11CH3]2– [H]+ is a Brønsted acid. Upon contact with water, [B12X11CH3]2–[H3O] + is formed, which can be detected by the characteristic "umbrella mode" of the coordinated hydronium ion (H3O) + (cf. Figs. 8 and 9).[4] In addition, the fragment ions [B12Br11]– and [B12Cl11]– were deposited on surfaces by the ion soft-landing method, where they reacted with the alkyl chains of organic compounds. The attachment to the nonpolar alkyl chains occurred selectively in the presence of much more reactive functional groups such as aromatic and ester groups. This surprising preference for C–H groups as reactants may be related to the fact that hydrophobic alkyl groups are oriented towards the vacuum in such surface layers. The superelectrophilic anions react directly at the vacuum interface with C–H groups of the phthalate alkyl groups according to the mechanism for methane binding shown in Figure 8.[4]
Reactions with anions
In the gas phase, ion-ion reactions can usually only take place between ions of opposite polarity - i.e. between anion and cation. The reaction partners attract each other by long-range Coulomb force, and the complementary reactivities of the nucleophilic anion and the electrophilic cation cause the formation of a stable bond. On the other hand, an anion usually has no affinity to bind to other anions for two reasons. Firstly, the large Coulomb barrier keeps ions of the same polarity at a distance and secondly, there is no thermodynamic driving force between two nucleophiles to form a bond. However, the strong electrophilicity of the [B12X11]– ions creates such a driving force, which enables to form stable bonds to some anions which creates highly charged ions. However, the repulsive electrostatic interactions are long range and therefore cannot be overcome in the gas phase by these electrophilic anions. Thus, the anions are kept at a distance and cannot react with each other. To circumvent this problem, the corresponding anions were selectively brought together on grounded surfaces using the ion soft-landing method. For example, the highly reactive fragment anion [B12I11]– and the stable dianion [B12I12]2– was used to create the trianion [B12I11-I-B12I11]3–.[5]
Variants
By exchanging the substituents (X), different variants of the closo-dodecaborate precursors and thus also of the fragment anions can be generated. So far, five variants have been produced experimentally in the gas phase and their reactivity investigated: The fragment anions [B12X11]– with substituents X = F, Cl, Br, I and CN.[3]
The nature of the substituents significantly influences the electrophilicity of the fragment anion. The thermodynamic stability of the precursor dianion seems to play an important role. A qualitative general guideline is:
The more electronically stable the precursor dianion, the more electrophilic the corresponding monoanion generated by gas-phase fragmentation.[1]
Table 1 provides an overview of the reactions possible at room temperature between the different noble gases and experimentally accessible variants of the superelectrophilic anions. Furthermore, it shows how the reactivity of the variants is related to the electronic stability of the precursor and to the calculated atomic charge of the vacant boron.
Table 1: Reactions of the variants with the different noble gases at room temperature in relation to precursor stability and atomic charge of the vacant boron (the latter determined using the Natural Population Analysis (NPA) method).[1][14]
| [B12F11]– | [B12I11]– | [B12Cl11]– | [B12Br11]– | [B12(CN)11]– | |
|---|---|---|---|---|---|
| Ne | ╳ | ╳ | ╳ | ╳ | ╳ |
| Ar | ╳ | ╳ | ╳ | ╳ | ✓ |
| Kr | ╳ | ╳ | ✓ | ✓ | ✓ |
| Xe | ✓ | ✓ | ✓ | ✓ | ✓ |
| Electronic stability of the precursor
(in eV) |
1,9 | 2,9 | 3,0 | 3,2 | 5,3 |
| Atomic charge of vacant boron
(in e) |
+ 0,45 | + 0,66 | + 0,64 | + 0,64 | + 0,82 |
It can be seen that the reactivities of [B12Cl11]– and [B12Br11]– can be classified as similar, while [B12F11]– and [B12I11]– are less reactive. The reduced noble gas binding strength can be attributed to a less electrophilic vacant boron atom in the case of X = F, because the atomic charge of the vacant boron calculated using the Natural Population Analysis (NPA) method is + 0.45 e, while it is much larger for the other halogens (for X = Cl, for example, + 0.64 e). In the case of X = I, the boron atom is similarly electrophilic as for X = Cl and X = Br, but the sterically very demanding iodine ligands are a hindrance for bond formation.[3]
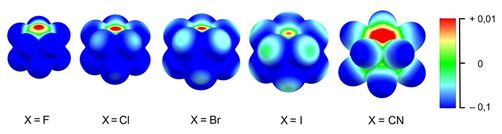
In [B12(CN)11]–, however, the vacant boron atom with an atomic charge of + 0.82 e is significantly more electrophilic than those of the halogenated variants (cf. Fig. 11).
Figure 11 shows the electrostatic potential on the molecular surface of the different [B12X11]– variants, illustrating the different reactivities of the vacant boron atoms, which are influenced on the one hand by the strength of the electron deficiency (positive electrostatic potential shown in red) and on the other hand by the accessibility of the electrophilic center. The latter depends significantly on the size of the substituents X, since these contribute to a spatial shielding of the vacant boron atom.
It should be noted, however, that the reactivities depend to a large extent on the type of reaction partner used. Expressed in a simplified way, the variants have different preferences with regard to their nucleophilic reaction partners. Furthermore, if a variant is more reactive towards a particular reaction partner than another variant, this does not mean that it is universally more reactive. For example, [B12Cl11]– binds noble gases more strongly than [B12F11]–, but the latter binds CO and N2 more strongly. This difference can be explained by the fact that the binding strength to noble gases is dominated by the electrophilicity of the vacant boron atom, whereas the binding to N2 or CO is significantly influenced by the formation of π-backbonds (see section Reactions with diatomic molecules). During π-backbonding, electron transfer occurs from occupied molecular orbitals of the [B12X11] – to the antibonding π-orbitals of the N2 or CO, respectively. However, since a lower electrophilicity of the vacant boron atom is accompanied by energetically higher lying occupied orbitals and thus an increased ability to donate electron density, the weakest noble gas binder is simultaneously the strongest N2 and CO binder among the superelectrophilic anions, and vice versa.[3]
Theoretical studies / computer simulations
Complementary to the described experimental studies, the reactivity of superelectrophilic anions has also been extensively investigated by theoretical "computational chemistry" methods. The noble gas-boron bond has been analyzed both in combined experimental-theoretical studies[1][2][6][3] and in independent theoretical studies.[15] For this purpose, methods such as Quantum Theory of Atoms in Molecules (QTAIM) and Energy Decomposition Analysis (EDA) have been used. The possibility of stabilizing compounds of the superelectrophilic anions with noble gases in the condensed phase has also been discussed and theoretically investigated.[16]
In addition, other theoretical studies are concerned with reactivity toward H2[17] or with electrophilic anions not accessible experimentally so far: These include beryllium-containing superelectrophilic dianions[18] and the anions [B12(BO)11]– and [B12(OBO)11]–.[19]
Scientific relevance and potential applications
The extraordinary reactivity of superelectrophilic anions enables them to form bonds to very unreactive atoms or molecules. Such reactions between superelectrophilic anions and unreactive substances are of interest in many respects:
Exploring new reaction mechanisms for direct functionalization of unreactive resources:
Many natural resources such as N2, CO2 or small hydrocarbons are very inert due to the stable bonds within the molecules. This prevents efficient utilization of these resources, which would be of high ecological and economic interest. With the help of superelectrophilic anions, new knowledge can be gained about mechanisms of direct functionalization of such unreactive compounds, which could potentially be helpful in the future searching for methods to utilize unreactive resources.[3][4]
Generation of exotic compounds for fundamental chemical research:
Reactions with [B12X11]– anions enable the preparation of a variety of exotic compounds that are of interest for fundamental chemical research. These include, for example, noble gas-containing molecular anions[1][2][6] or highly charged cluster ions[5] that are usually not synthetically accessible. There has been some research on noble gas-containing molecular anions, but most of it is purely theoretical. In contrast, noble gas-containing anions of the type [B12X11-NG]– (NG = noble gas) can actually be prepared experimentally, not only at temperatures near absolute zero (0 Kelvin) as required for weakly bound complexes, but also at room temperature (298 Kelvin). Moreover, they are comparatively robust against attack by nucleophiles such as water. At room temperature, small amounts of nucleophiles such as water can hardly be avoided in vacuum instruments and tend to decompose noble gas-containing reaction products by substitution reactions. However, this problem does not play a major role in the case of the noble gas-containing anions of the type [B12X11-NG]– described here.[1][2]
Generation of novel substances as prototypes for potential drugs:
[B12X11]– ions can be directly bound to larger organic structures to produce entirely new boron-containing organic molecules in this way.[4] Such novel molecules could represent interesting reagents in the future, for example, for cancer drugs in the context of boron neutron capture therapy.[7] For this, however, the synthesis methods would first have to be improved so that significantly higher yields can be achieved in the condensed phase.
Generation of permanent ions for electrospray mass spectrometry analyses:
By binding [B12X11]– to neutral, nonpolar molecules, these can be converted into permanent ions. This has the advantage of opening up new perspectives for the analysis of these nonpolar molecules with electrospray mass spectrometry, which would not be possible without the binding to the [B12X11]– ions.
Research locations
Research on superelectrophilic anions in the gas phase has its origin at the University of Bremen and at the Wilhelm Ostwald Institute (WOI) of the University of Leipzig (Germany ). The main focus is currently at WOI, where most of the experimental studies take place.[20][21]
U.S. research groups from Pacific Northwest National Laboratory (PNNL) in Richland and Purdue University in West Lafayette have also made important experimental contributions to the study of superelectrophilic anions through collaborations with WOI.
The precursors, which are used to generate the electrophilic anions, are mostly synthesized at the Bergische Universität Wuppertal (Germany). Theoretical studies on this class of compounds have already been carried out by many groups around the world - such as in the USA, China, India or Germany.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 Mayer, Martin; Lessen, Valentin van; Rohdenburg, Markus; Hou, Gao-Lei; Yang, Zheng; Exner, Rüdiger M.; Aprà, Edoardo; Azov, Vladimir A. et al. (2019-04-23). "Rational design of an argon-binding superelectrophilic anion" (in en). Proceedings of the National Academy of Sciences 116 (17): 8167–8172. doi:10.1073/pnas.1820812116. ISSN 0027-8424. PMID 30952786.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Rohdenburg, Markus; Mayer, Martin; Grellmann, Max; Jenne, Carsten; Borrmann, Tobias; Kleemiss, Florian; Azov, Vladimir A.; Asmis, Knut R. et al. (2017). "Superelectrophilic Behavior of an Anion Demonstrated by the Spontaneous Binding of Noble Gases to [B12Cl11−"] (in en). Angewandte Chemie International Edition 56 (27): 7980–7985. doi:10.1002/anie.201702237. ISSN 1521-3773. PMID 28560843. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201702237.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Mayer, Martin; Rohdenburg, Markus; Kawa, Sebastian; Horn, Francine; Knorke, Harald; Jenne, Carsten; Tonner, Ralf; Asmis, Knut R. et al. (2021). "Relevance of π-Backbonding for the Reactivity of Electrophilic Anions [B12X11− (X=F, Cl, Br, I, CN)"] (in en). Chemistry – A European Journal 27 (40): 10274–10281. doi:10.1002/chem.202100949. ISSN 1521-3765. PMID 34014012.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Warneke, Jonas; Mayer, Martin; Rohdenburg, Markus; Ma, Xin; Liu, Judy K. Y.; Grellmann, Max; Debnath, Sreekanta; Azov, Vladimir A. et al. (2020-09-22). "Direct functionalization of C−H bonds by electrophilic anions" (in en). Proceedings of the National Academy of Sciences 117 (38): 23374–23379. doi:10.1073/pnas.2004432117. ISSN 0027-8424. PMID 32878996. Bibcode: 2020PNAS..11723374W.
- ↑ 5.0 5.1 5.2 5.3 Yang, Fangshun; Behrend, K. Antonio; Knorke, Harald; Rohdenburg, Markus; Charvat, Ales; Jenne, Carsten; Abel, Bernd; Warneke, Jonas (2021). "Anion–Anion Chemistry with Mass-Selected Molecular Fragments on Surfaces" (in en). Angewandte Chemie International Edition 60 (47): 24910–24914. doi:10.1002/anie.202109249. ISSN 1521-3773. PMID 34523217.
- ↑ 6.0 6.1 6.2 6.3 6.4 Mayer, Martin; Rohdenburg, Markus; Lessen, Valentin van; Nierstenhöfer, Marc C.; Aprà, Edoardo; Grabowsky, Simon; Asmis, Knut R.; Jenne, Carsten et al. (2020-04-23). "First steps towards a stable neon compound: observation and bonding analysis of [B12(CN)11Ne]−" (in en). Chemical Communications 56 (33): 4591–4594. doi:10.1039/D0CC01423K. ISSN 1364-548X. PMID 32207481.
- ↑ 7.0 7.1 Hosmane, Narayan S. (2011) (in en). Boron Science - New Technologies and Applications. Routledge. ISBN 9781439826621.
- ↑ 8.0 8.1 Roithová, Jana (2011-04-04). "Superelectrophilic chemistry in the gas phase" (in en). Pure and Applied Chemistry 83 (8): 1499–1506. doi:10.1351/PAC-CON-10-10-17. ISSN 0033-4545. http://pac.iupac.org/publications/pac/83/8/1499/.
- ↑ Olah, George A. (1993). "Superelektrophile" (in de). Angewandte Chemie 105 (6): 805–827. doi:10.1002/ange.19931050604. ISSN 1521-3757. Bibcode: 1993AngCh.105..805O. https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.19931050604.
- ↑ Dieterle, Martin; Harvey, Jeremy N.; Heinemann, Christoph; Schwarz, Joseph; Schröder, Detlef; Schwarz, Helmut (1997-10-17). "Equilibrium studies of weakly bound Fe(L)+ complexes with L = Xe, CO2, N2 and CH4" (in en). Chemical Physics Letters 277 (5): 399–405. doi:10.1016/S0009-2614(97)00898-1. ISSN 0009-2614. Bibcode: 1997CPL...277..399D. https://www.sciencedirect.com/science/article/pii/S0009261497008981.
- ↑ Schröder, Detlef; Schwarz, Helmut; Hrušák, Jan; Pyykkö, Pekka (1998-02-23). "Cationic Gold(I) Complexes of Xenon and of Ligands Containing the Donor Atoms Oxygen, Nitrogen, Phosphorus, and Sulfur". Inorganic Chemistry 37 (4): 624–632. doi:10.1021/ic970986m. ISSN 0020-1669. https://doi.org/10.1021/ic970986m.
- ↑ Knapp, Charles (in en). Comprehensive Inorganic Chemistry II, Vol. 1. Amsterdam: Elsevier. pp. 651–679.
- ↑ 13.0 13.1 Warneke, Jonas; Dülcks, Thomas; Knapp, Carsten; Gabel, Detlef (2011-03-16). "Collision-induced gas-phase reactions of perhalogenated closo-dodecaborate clusters – a comparative study" (in en). Physical Chemistry Chemical Physics 13 (13): 5712–5721. doi:10.1039/C0CP02386H. ISSN 1463-9084. PMID 21311775. Bibcode: 2011PCCP...13.5712W. https://pubs.rsc.org/en/content/articlelanding/2011/cp/c0cp02386h.
- ↑ Warneke, Jonas; Hou, Gao-Lei; Aprà, Edoardo; Jenne, Carsten; Yang, Zheng; Qin, Zhengbo; Kowalski, Karol; Wang, Xue-Bin et al. (2017-10-18). "Electronic Structure and Stability of [B12X122– (X = F–At): A Combined Photoelectron Spectroscopic and Theoretical Study"]. Journal of the American Chemical Society 139 (41): 14749–14756. doi:10.1021/jacs.7b08598. ISSN 0002-7863. PMID 28933868. https://doi.org/10.1021/jacs.7b08598.
- ↑ Wöhner, Kevin; Wulf, Toshiki; Vankova, Nina; Heine, Thomas (2021-06-10). "Strong Binding of Noble Gases to [B12X11−: A Theoretical Study"]. The Journal of Physical Chemistry A 125 (22): 4760–4765. doi:10.1021/acs.jpca.1c01909. ISSN 1089-5639. PMID 34036781. Bibcode: 2021JPCA..125.4760W. https://doi.org/10.1021/acs.jpca.1c01909.
- ↑ Rohdenburg, Markus; Azov, Vladimir A.; Warneke, Jonas (2020). "New Perspectives in the Noble Gas Chemistry Opened by Electrophilic Anions". Frontiers in Chemistry 8: 580295. doi:10.3389/fchem.2020.580295. ISSN 2296-2646. PMID 33282830. Bibcode: 2020FrCh....8.1015W.
- ↑ Wulf, Toshiki; Warneke, Jonas; Heine, Thomas (2021-08-23). "B12X11(H2)−: exploring the limits of isotopologue selectivity of hydrogen adsorption" (in en). RSC Advances 11 (46): 28466–28475. doi:10.1039/D1RA06322G. ISSN 2046-2069. PMID 35478551. Bibcode: 2021RSCAd..1128466W.
- ↑ Joshi, Meenakshi; Ghanty, Tapan K. (2019-11-26). "Quantum chemical prediction of a superelectrophilic dianion and its binding with noble gas atoms" (in en). Chemical Communications 55 (95): 14379–14382. doi:10.1039/C9CC08049J. ISSN 1364-548X. PMID 31720611. https://pubs.rsc.org/en/content/articlelanding/2019/cc/c9cc08049j.
- ↑ Xu, Jianzhi; Li, Mengyang; Xu, Song; Pei, Gerui; Zhao, Xintian; Kong, Chuncai; Yang, Zhimao; Yang, Tao et al. (2021). "Stable Noble Gas Compounds Based on Superelectrophilic Anions [B12(BO)11− and [B12(OBO)11]−"] (in en). ChemPhysChem 22 (21): 2240–2246. doi:10.1002/cphc.202100391. ISSN 1439-7641. PMID 34402158. https://onlinelibrary.wiley.com/doi/abs/10.1002/cphc.202100391.
- ↑ "Homepage of Dr. J. Warneke, Wilhelm-Ostwald-Institut, Universität Leipzig". https://woi.chemie.uni-leipzig.de/start/nfg-dr-warneke/.
- ↑ "Homepage of Prof. K. Asmis, Wilhelm-Ostwald-Institut, Universität Leipzig". https://www.uni-leipzig.de/professur-fuer-physikalische-chemie-1.html.
External links
- Homepage of Dr. Jonas Warneke, Wilhelm-Ostwald-Institute at Leipzig University (Germany)
- Homepage of Prof. Carsten Jenne, University of Wuppertal (Germany)
 |