Chemistry:Tetraazidomethane
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tetraazidomethane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C(N 3) 4 | |||
| Molar mass | 180.095 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Boiling point | ~165 °C (estimate) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
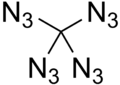
Tetraazidomethane, C(N
3)
4, is a colorless, highly explosive liquid. Its chemical structure consists of a carbon atom covalently bonded to four azide functional groups.
Synthesis
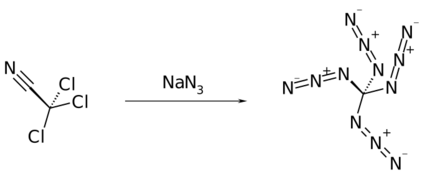
It was first prepared by Klaus Banert in 2006 by reaction of trichloroacetonitrile with sodium azide.[1]
Uses
As with other polyazides, tetraazidomethane has interest as a high-energy-density material with potential uses in explosives, propellants, or fireworks.[2] Silicon tetraazide is also a known compound.
Reactions
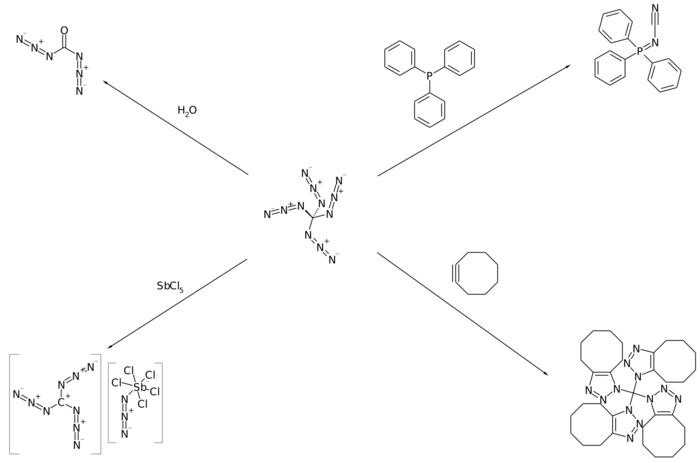
Banert has reported that tetraazidomethane participates in a number of reactions including hydrolysis, cycloaddition reactions with alkenes and alkynes, and reaction with phosphines to form phosphazenes.[1]
References
- ↑ 1.0 1.1 "The Exciting Chemistry of Tetraazidomethane", Klaus Banert, Young-Hyuk Joo, Tobias Ruffer, Bernhard Walfort, and Heinrich Lang, Angew. Chem. Int. Ed. 2007, 46, 1168–1171. doi:10.1002/anie.200603960
- ↑ "Tetraazidomethane: Chemistry with a Bang", Chemical & Engineering News, Dec. 18, 2006, 46.
 |