Chemistry:Tetraphenylethylene
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1′,1′′,1′′′-Ethenetetrayltetrabenzene | |||
| Other names
1,1,2,2-Tetraphenylethene
Tetraphenylethylene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 789087 | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C26H20 | |||
| Molar mass | 332.446 g·mol−1 | ||
| Appearance | yellow solid | ||
| Density | 1.088 g/cm3 | ||
| Melting point | 224 to 225 °C (435 to 437 °F; 497 to 498 K) | ||
| Boiling point | 424 °C (795 °F; 697 K)[1] | ||
| Hazards | |||
| Flash point | 206.2 °C (403.2 °F; 479.3 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
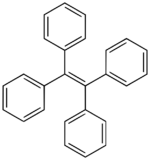
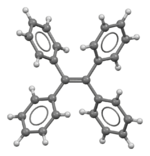
Tetraphenylethene (TPE) is an organic chemical compound with the formula Ph2C=CPh2, where Ph = phenyl (C6H5). It has been described as a yellow solid, but single crystals are colorless. The molecule is crowded such that all four phenyl groups are twisted out of the plane defined by the center six carbon atoms.[2] Tetraphenylethene is used as a precursor to other organic compounds, often in the area of supramolecular chemistry.
Synthesis
Tetraphenylethene can be synthesized from diphenyldichloromethane.[3][4]
References
- ↑ Lewis, Irwin C.; T. Edstrom (1963). "Thermal Reactivity of Polynuclear Aromatic Hydrocarbons". The Journal of Organic Chemistry 28 (8): 2050–2057. doi:10.1021/jo01043a025.
- ↑ Hoekstra, A.; Vos, A. (1975). "The Crystal and Molecular Structures of Tetraphenylhydrazine and Related Compounds at –160°C. II. The Crystal Structures of Tetraphenylethylene (TPE) and Diphenylaminotriphenylmethane (DTM)". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 31 (6): 1716–1721. doi:10.1107/S0567740875005973.
- ↑ Inaba, S (1982). "Metallic nickel as a reagent for the coupling of aromatic and benzylic halides". Tetrahedron Letters 23 (41): 4215–4216. doi:10.1016/S0040-4039(00)88707-9.
- ↑ Robert E. Buckles George M. Matlack (1951). "Tetraphenylethylene". Organic Syntheses 31: 104. doi:10.15227/orgsyn.031.0104.
 |