Chemistry:Thiosulfoxide
A thiosulfoxide or thiothionyl compound is a chemical compound containing a sulfur to sulfur double bond, with the formula (R–)(R'–)S=S, where R and R' represent any group (typically fluorine, chlorine, alkoxy, alkyl, aryl or other organyl residues. The thiosulfoxide has a molecular shape known as trigonal pyramidal. Its coordination is also trigonal pyramidal. The point group of the thiosulfoxide is Cs. A 1982 review concluded that there was as yet no definitive evidence for the existence of stable thiosulfoxides [1] which can be attributed to the double bond rule which states that elements of period 3 and beyond do not form multiple bonds. The related sulfoxides of the type (R–)(R'–)S=O are very common. Many compounds containing a sulfur-sulfur double bond have been reported in the past although only a few verified classes of actually stable compounds exist, closely related to thiosulfoxides.
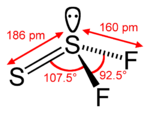
Sulfur-sulfur double bonds can be stabilized with electron-withdrawing groups in so-called thionosulfites of the type (R–O–)(R'–O–)S=S. These compounds can be prepared by reaction of diols with disulfur dichloride. Sulfur halides such as disulfur dichloride, Cl–S–S–Cl, can convert to the branched isomer thiothionyl chloride, Cl
2S=S; disulfur difluoride exists as an equilibrium mixture with thiothionyl fluoride, F
2S=S, which is thermodynamically more stable. These disulfide isomerizations are occasionally studied in silico.[2]
N-(Thiosulfinyl)amines of the type R–N=S=S are another group of stable compounds containing a S=S bond. The first such compound was prepared in 1974 reaction of the nitroso compound N,N-dimethyl-p-nitrosoaniline with tetraphosphorus decasulfide. Heating to 200 °C extrudes sulfur in this compound and forms the corresponding azo compound. Disulfur monoxide S=S=O is stable at 20 °C for several days.
Occasionally thiosulfates are depicted as having a S=S unit but the sulfur-sulfur bond in it is in fact a single bond.
References
- ↑ Gerald W. Kutney; Kenneth Turnbull (1982). "Compounds containing the sulfur-sulfur double bond". Chem. Rev. 82 (4): 333–357. doi:10.1021/cr00050a001.
- ↑ Ralf Steudel; Yana Drozdova; Karol Miaskiewicz; Roland H. Hertwig; Wolfram Koch (1997). "How Unstable are Thiosulfoxides? An ab Initio MO Study of Various Disulfanes RSSR (R = H, Me, Pr, All), Their Branched Isomers R2SS, and the Related Transition States". J. Am. Chem. Soc. 119 (8): 1990–1996. doi:10.1021/ja9624026.
 |
