Chemistry:Disulfur difluoride
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
fluorosulfanyl thiohypofluorite
| |||
| Other names
Difluorodisulfane[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| S 2F 2 | |||
| Molar mass | 102.127 g/mol | ||
| Melting point | −133 °C (−207 °F; 140 K) | ||
| Boiling point | 15 °C (59 °F; 288 K) | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Disulfur difluoride is an inorganic compound with the chemical formula S
2F
2. It is a halide of sulfur.
Structure
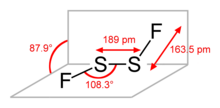
Disulfur difluoride has a chain structure F–S–S–F. The angle between the Fa
–S–S and S–S–Fb
planes is 87.9°, while the angles of Fa
–S–S and S–S–Fb
are equivalent, and are equal to 108.3°. Both S–F bonds are equivalent and their length is 163.5 pm, while the length of the S–S bond is 189 pm. This structure is referred to as gauche, and is similar to H
2O
2.
There is a branched isomer of disulfur difluoride, thiothionyl fluoride, with the structure S=SF
2.
Synthesis
Silver(II) fluoride can fluorinate sulfur in a strictly dry container at 125 °C (257 °F; 398 K), and the reaction produces FS–SF:[2]
Reactions
Disulfur difluoride undergoes intramolecular rearrangement in the presence of fluorides of alkali metals, yielding the isomer S=SF2:[3]
- FS–SF → S=SF
2
- Decomposing to sulfur tetrafluoride and sulfur when heated to 180 °C:
- 2 S
2F
2 → SF
4 + 3 S
- Reacting with sulfuric acid at 80 °C:
- S
2F
2 + 3 H
2SO
4 → 5 SO
2 + 2 HF + 2 H
2O
- Reacting with sodium hydroxide:
- Reacting with oxygen at high pressure, using nitrogen dioxide as a catalyst:
References
- ↑ "Difluorodisulfane". https://pubchem.ncbi.nlm.nih.gov/compound/Difluorodisulfane.
- ↑ Davis, R.Wellington; Firth, Steven (1991). "The microwave spectrum of the chain isomer of disulfur difluoride: FS-SF". Journal of Molecular Spectroscopy 145 (2): 225. doi:10.1016/0022-2852(91)90109-N.
- ↑ Davis, R.Wellington (1986). "The microwave spectrum of the pyramidal isomer of disulfur difluoride: S=SF2". Journal of Molecular Spectroscopy 116 (2): 371–383. doi:10.1016/0022-2852(86)90134-7.
- ↑ Справочник химика / Редкол.: Никольский Б.П. и др.. — 3-е изд., испр. — Л.: Химия, 1971. — Т. 2. — 1168 с. (in Russian)
- ↑ Химическая энциклопедия / Редкол.: Кнунянц И.Л. и др.. — М.: Советская энциклопедия, 1995. — Т. 4. — 639 с. — ISBN:978-5-85270-092-6 (in Russian)
- ↑ Лидин Р.А. и др. Химические свойства неорганических веществ: Учеб. пособие для вузов. — 3-е изд., испр. — М.: Химия, 2000. — 480 с. — ISBN:978-5-7245-1163-6 (in Russian)
 |



