Chemistry:Thiourea dioxide
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Amino(imino)methanesulfinic acid | |
| Other names
Thiourea dioxide, DegaFAS, Reducing Agent F, Depilor, Formamidine Sulfinic Acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CH4N2O2S | |
| Molar mass | 108.12 g·mol−1 |
| Appearance | White powder |
| Melting point | 126 °C (259 °F; 399 K) |
| 3.0 g/100 mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
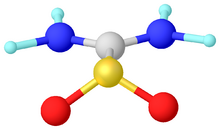
Thiourea dioxide or thiox is an organosulfur compound that is used in the textile industry.[1] It functions as a reducing agent.[2] It is a white solid, and exhibits tautomerism.[citation needed]
Structure
The structure of thiourea dioxide depends on its environment. Crystalline and gaseous thiourea dioxide adopts a structure with C2v symmetry. Selected bond lengths: S-C = 186, C-N = 130, and S-O = 149 pm. The sulfur center is pyramidal. The C-S bond length is more similar to that of a single bond. For comparison, the C=S bond in thiourea is 171 pm.[3][4] The long C-S bond indicates the absence of C=S character. Instead the bonding is described with a significant contribution from a dipolar resonance structure with multiple bonding between C and N. One consequence of this bonding is the planarity of the nitrogen centers.[5] In the presence of water or DMSO, thiourea dioxide converts to the tautomer, a sulfinic acid, (H2N)HN=CS(O)(OH), named formamidine sulfinic acid.[5]
Synthesis
Thiourea dioxide was first prepared in 1910 by the English chemist Edward de Barry Barnett.[6]
Thiourea dioxide is prepared by the oxidation of thiourea with hydrogen peroxide.[7]
- (NH2)2CS + 2H2O2 → (NH)(NH2)CSO2H + 2H2O
The mechanism of the oxidation has been examined.[8] An aqueous solution of thiourea dioxide has a pH about 6.5 at which thiourea dioxide is hydrolyzed to urea and sulfoxylic acid. It has been found that at pH values of less than 2, thiourea and hydrogen peroxide react to form a disulfide species. It is therefore convenient to keep the pH between 3 and 5 and the temperature below 10 °C.[9] It can also be prepared by oxidation of thiourea with chlorine dioxide.[10] The quality of the product can be assessed by titration with indigo.[7]
Uses
Thiourea dioxide is used in reductive bleaching in textiles.[11] Thiourea dioxide has also been used for the reduction of aromatic nitroaldehydes and nitroketones to nitroalcohols.[12]
References
- ↑ Fischer, Klaus (2003). "Textile Auxiliaries". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_227. ISBN 9783527303854. OCLC 55738480. https://books.google.com/books?id=YH1UAAAAMAAJ. Retrieved 2022-06-18.
- ↑ Milne, George W. A. (11 July 2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. Hoboken, New Jersey, USA: Wiley-Interscience. doi:10.1002/0471736627.ch1. ISBN 9780471735182. OCLC 57392953. https://books.google.com/books?id=9vNTAAAAMAAJ. Retrieved 18 June 2022.
- ↑ Sullivan, R. A. L.; Hargreaves, A. (1962). "The Crystal and Molecular Structure of Thiourea Dioxide". Acta Crystallographica 15 (7): 675–682. doi:10.1107/S0365110X62001851.
- ↑ Chen, I-C.; Wang, Y. (1984). "Reinvestigation of the Structure of Thiourea S,S‐Dioxide, CH4N2O2S". Acta Crystallographica 40 (11): 1937–1938. doi:10.1107/S010827018401012X.
- ↑ Jump up to: 5.0 5.1 Makarov, S. V. (2001). "Recent Trends in the Chemistry of Sulfur-Containing Reducing Agents". Russian Chemical Reviews 70 (10): 885–895. doi:10.1070/RC2001v070n10ABEH000659. Bibcode: 2001RuCRv..70..885M.
- ↑ Barnett first prepared thiourea dioxide ("aminoiminomethanesulphinic acid") by oxidizing thiourea ("thiocarbamide") with hydrogen peroxide ("hydrogen dioxide"). See: Barnett, Edward de Barry (1910) "The action of hydrogen dioxide on thiocarbamides," Journal of the Chemical Society, Transactions, 97 : 63–65.
- ↑ Jump up to: 7.0 7.1 D. Schubart "Sulfinic Acids and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_461
- ↑ Hoffmann, Michael; Edwards, John O. (1977). "Kinetics and Mechanism of the Oxidation of Thiourea and N,N'-dialkylthioureas by Hydrogen Peroxide". Inorganic Chemistry 16 (12): 3333–3338. doi:10.1021/ic50178a069.
- ↑ US patent 2783272, James H. Young, "PRODUCTION OF FORMAMIDINE SULFINIC ACID", issued 1957-2-26
- ↑ Rábai, G.; Wang, R. T.; Kustin, Kenneth (1993). "Kinetics and mechanism of the oxidation of thiourea by chlorine dioxide" International Journal of Chemical Kinetics. Volume 25: 53–62. doi:10.1002/kin.550250106
- ↑ Hebeish, A.; El-Rafie, M. H.; Waly, A.; Moursi, A. Z. (1978). "Graft copolymerization of vinyl monomers onto modified cotton. IX. Hydrogen peroxide–thiourea dioxide redox system induced grafting of 2-methyl-5-vinylpyridine onto oxidized celluloses". Journal of Applied Polymer Science 22 (7): 1853–1866. doi:10.1002/app.1978.070220709.
- ↑ Sambher, Shikha; Baskar, Chinnappan; Dhillon, Ranjit S. (22 May 2009). "Chemoselective reduction of carbonyl groups of aromatic nitro carbonyl compounds to the corresponding nitroalcohols using thiourea dioxide". Arkivoc 2009 (10): 141–145. doi:10.3998/ark.5550190.0010.a14.
 |


