Chemistry:Transition metal thioether complex

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.
Dimethylsulfide complexes
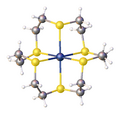
As the simplest thioether, dimethyl sulfide forms complexes that are illustrative of the class.[2] Well characterized derivatives include cis-[TiCl4L2], VCl3L2, NbCl5L, NbCl4L2, Cr(CO)5L, CrCl3L3, RuCl2L4, RuCl3L3, RhCl3L3, cis- and trans-[IrCl4L3]-, cis-MCl2L2 (M = Pd, Pt), [PtCl3L]−, cis- and trans-[PtCl4L2] (L = SMe2). With respect to donor properties, dimethyl sulfide is a soft ligand with donor properties weaker than phosphine ligands.[3] Such complexes are generally prepared by treating the metal halide with the thioether. Chloro(dimethyl sulfide)gold(I) can however be prepared by redox reaction of elemental gold and DMSO in the presence of hydrochloric acid.[4]
Stereochemistry
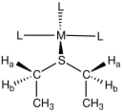
Thioether complexes feature pyramidal sulfur centers. Typical C-S-C angles are near 99° in both free thioethers and their complexes. The C-S distance in dimethylsulfide is 1.81 Å, which is also unaffected in its complexes.[5] The stereochemistry of thioether complexes have been extensively studied.[6] Unsymmetrical thioethers, e.g., SMeEt, are prochiral ligands, and their complexes are chiral. One example is [Ru(NH3)5(SMeEt)]2+. The complex cis-VOCl2(SMeEt)2 exists as meso- and a pair of enantiomers.[7] In complexes of thioethers of the type S(CH2R)2 (R ≠ H), the methylene protons are diastereotopic. Examination of the NMR spectra of such complexes reveal that they undergo inversion at sulfur, without dissociation of the M-S bond.[8]
Thioether as a bridging ligand
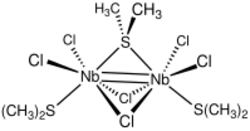
Unlike ethers, thioethers occasionally serve as bridging ligands. The complexes Nb2Cl6(SMe2)3 is one such example. It adopts a face-sharing bioctahedral structure with a Nb(III)=Nb(III) bond, spanned by two chloride and one dimethylsulfide ligands. The complex Pt2Me4(μ-SMe2)2 is a source of "PtMe2".[10]
Complexes chelating thioether ligands
Thiacrown ligands are analogous to crown ethers. The best studied thiacrown ligands have the formula (SCH2CH2)n (n = 3,4,5,6). The tridentate tri-thioether 9-ane-S3 forms extensive families of complexes of the type M(9-ane-S3)L3 and [M(9-ane-S3)2]2+. Examples of Cu(II)-thioether complexes were prepared from 14-ane-S4[13] and 15-ane-S5.[11] The hexadentate ligand 18-ane-6 also forms extensive family of complexes, including unusual examples of Pd(III) and Ag(II).[14] Examples of homoleptic complexes [M(SR2)6]n+ are otherwise rare.
Occurrence
Thioether complexes in nature arise from coordination of the sulfur substituent found in the amino acid methionine. One of the axial ligands in cytochrome c is illustrative. Methionine sulfur weakly binds to copper in azurin.
References
- ↑ Krogh-Jespersen, Karsten; Zhang, Xiaohua; Ding, Yanbo; Westbrook, John D.; Potenza, Joseph A.; Schugar, Harvey J. (1992). "Molecular and Electronic Structures of Pentaammineruthenium(II)-Thioether Complexes. The Nature of Ru(II)-S Back Bonding Elucidated by Structural, Electronic Spectral, and Molecular Orbital Studies". Journal of the American Chemical Society 114 (11): 4345–4353. doi:10.1021/ja00037a047.
- ↑ Murray, Stephen G.; Hartley, Frank R. (1981). "Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes". Chemical Reviews 81 (4): 365–414. doi:10.1021/cr00044a003.
- ↑ Lever, A. B. P. (1990). "Electrochemical Parametrization of Metal Complex Redox Potentials, Using the Ruthenium(III)/Ruthenium(II) Couple to Generate a Ligand Electrochemical Series". Inorganic Chemistry 29 (6): 1271–1285. doi:10.1021/ic00331a030.
- ↑ Mueller, Thomas E.; Green, Jennifer C.; Mingos, D. Michael P.; McPartlin, Jennifer C.; Whittingham, Conrad; Williams, David J.; Woodroffe, Thomas M. (1998). "Complexes of gold(I) and platinum(II) with polyaromatic phosphine ligands". J. Organomet. Chem. 551 (1-2): 313. doi:10.1016/S0022-328X(97)00522-6.
- ↑ Iijima, T.; Tsuchiy, S.; Kimura, M. (1977). "The Molecular Structure of Dimethyl Sulfide". Bull. Chem. Soc. Jpn. 50: 2564. doi:10.1246/bcsj.50.2564.
- ↑ Abel, Edward W.; Bhargava, Suresh K.; Orrell, Keith G. (2007). "The Stereodynamics of Metal Complexes of Sulfur-, Selenium-, and Tellurium-Containing Ligands". Progress in Inorganic Chemistry: 1–118. doi:10.1002/9780470166338.ch1.
- ↑ Matsuura, Masatoshi; Fujihara, Takashi; Nagasawa, Akira (2013). "Cis-Dichloridobis(ethyl methyl sulfide-κS)oxidovanadium(IV)". Acta Crystallographica Section E 69 (4): m209. doi:10.1107/S1600536813006703. PMID 23634004.
- ↑ Turley, Patricia C.; Haake, Paul. (1967). "Proton Magnetic Resonance Epectra of Platinum(II) Complexes. II. Cis- and trans- Bis(dialkyl sulfide)dichloroplatinum(II) Complexes. Mechanism of Inversion at Sulfur and Vicinal Platinum-Proton Couplings". Journal of the American Chemical Society 89 (18): 4617–4621. doi:10.1021/ja00994a009.
- ↑ Kakeya, Masaki; Fujihara, Takashi; Nagasawa, Akira (2006). "Di-μ-chloro-μ-(dimethyl sulfide)-bis[dichloro(dimethyl sulfide)niobium(III)]". Acta Crystallographica Section E 62 (3): m553–m554. doi:10.1107/S1600536806005149.
- ↑ Hill, Geoffrey S.; Irwin, Michael J.; Levy, Christopher J.; Rendina, Louis M.; Puddephatt, Richard J. (1998). "Platinum(II) Complexes of Dimethyl Sulfide". Inorganic Syntheses 32: 149–153. doi:10.1002/9780470132630.ch25. ISBN 9780470132630.
- ↑ 11.0 11.1 Janzen, Daron E.; Vanderveer, Donald G.; Mehne, Larry F.; Grant, Gregory J. (2010). "Ruthenium(II) Thiacrown Complexes: Synthetic, Spectroscopic, Electrochemical, DFT, and Single Crystal X-ray Structural Studies of [Ru([15]aneS5)Cl](PF6)". Inorganica Chimica Acta 364: 55–60. doi:10.1016/j.ica.2010.08.021.
- ↑ Musker, W. Kenneth (1992). "Coordination Chemistry of Bidentate Medium Ring Ligands (Mesocycles)". Coordination Chemistry Reviews 117: 133–57. doi:10.1016/0010-8545(92)80022-J.
- ↑ Pett, Virginia B.; Diaddario, Leonard L.; Dockal, Edward R.; Corfield, Peter W.; Ceccarelli, Christopher; Glick, Milton D.; Ochrymowycz, L. A.; Rorabacher, D. B. (1983). "Ring Size Effects on the structure of macrocyclic ligand complexes: Copper(II) complexes with 12-16-membered cyclic tetrathia ethers". Inorganic Chemistry 22 (24): 3661–3670. doi:10.1021/ic00166a033.
- ↑ Shaw, Jennifer L.; Wolowska, Joanna; Collison, David; Howard, Judith A. K.; McInnes, Eric J. L.; McMaster, Jonathan; Blake, Alexander J.; Wilson, Claire et al. (2006). "Redox Non-innocence of Thioether Macrocycles: Elucidation of the Electronic Structures of Mononuclear Complexes of Gold(II) and Silver(II)". Journal of the American Chemical Society 128 (42): 13827–13839. doi:10.1021/ja0636439. PMID 17044711.
 |