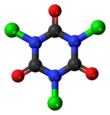
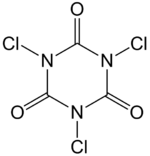
Chemistry:Trichloroisocyanuric acid
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,5-Trichloro-1,3,5-triazinane-2,4,6-trione | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 202022 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 240759 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2468 | ||
| |||
| |||
| Properties | |||
| C3Cl3N3O3 | |||
| Molar mass | 232.40 g·mol−1 | ||
| Appearance | Colorless solid | ||
| Density | 2.19 ± 0.1 g/cm3 | ||
| Melting point | 246 to 247 °C (475 to 477 °F; 519 to 520 K) | ||
| Boiling point | decomposes | ||
| 1.2% | |||
| Solubility in other solvents | Soluble in chlorocarbons, acetone, and acetonitrile | ||
| Structure | |||
| planar | |||
| 0 D | |||
| Hazards | |||
| Main hazards | lung irritant | ||
| GHS pictograms |   
| ||
| GHS Signal word | Warning | ||
| H272, H302, H319, H335, H410 | |||
| P210, P220, P221, P261, P264, P270, P271, P273, P280, P301+312, P304+340, P305+351+338, P312, P330, P337+313, P370+378, P391, P403+233, P405, P501 | |||
| Flash point | NA | ||
| Related compounds | |||
Related compounds
|
Cyanuric chloride Dichloroisocyanuric acid Tribromoisocyanuric acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trichloroisocyanuric acid is an organic compound with the formula (C3Cl3N3O3). It is used as an industrial disinfectant, bleaching agent and a reagent in organic synthesis.[1][2][3] This white crystalline powder, which has a strong "chlorine odour," is sometimes sold in tablet or granule form for domestic and industrial use.
Synthesis
Trichloroisocyanuric acid is prepared from cyanuric acid via a reaction with chlorine gas and trisodium cyanurate.[4]
Applications
The compound is a disinfectant, algicide and bactericide mainly for swimming pools and dyestuffs, and is also used as a bleaching agent in the textile industry. It is widely used in civil sanitation for pools and spas, preventing and curing diseases in animal husbandry and fisheries, fruit and vegetable preservation, wastewater treatment, as an algicide for recycled water in industry and air conditioning, in anti shrink treatment for woolens, for treating seeds and in organic chemical synthesis. It is used in chemical synthesis as an easy to store and transport chlorine gas source, it is not subject to hazardous gas shipping restrictions, and its reaction with hydrochloric acid produces relatively pure chlorine.[5]
Trichloroisocyanuric acid as used in swimming pools is easier to handle than chlorine gas. It dissolves slowly in water, but as it reacts, cyanuric acid concentration in the pool will build-up.
See also
- Comet (cleanser)
- Dichloroisocyanuric acid (Dichlor)
- Sodium dichloroisocyanurate
- Chlorine
References
- ↑ Hiegel, G. A. (2001). Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rt209. ISBN 0471936235.
- ↑ Barros, J. C. (2005). "Trichloroisocyanuric acid". Synlett 2005 (13): 2115–2116. doi:10.1055/s-2005-872237.
- ↑ Tilstam, Ulf; Weinmann, Hilmar (July 2002). "Trichloroisocyanuric Acid: A Safe and Efficient Oxidant". Organic Process Research & Development 6 (4): 384–393. doi:10.1021/op010103h.
- ↑ Chattaway, F. D.; Wadmore, J. Mello (1902). "XX.—The constitution of hydrocyanic, cyanic, and cyanuric acids". J. Chem. Soc., Trans. 81: 191–203. doi:10.1039/CT9028100191. https://zenodo.org/record/2128442.
- ↑ L. Lerner (2011). "Chlorine". Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling. CRC Press. ISBN 9780367383046.
External links
 |