Chemistry:Trifluoromethyl hypofluorite
From HandWiki
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trifluoromethyl hypofluorite | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| OCF4 | |||
| Molar mass | 104.004012 | ||
| Appearance | Colourless gas | ||
| Melting point | −213 °C (−351.4 °F; 60.1 K) | ||
| Boiling point | −95 °C (−139 °F; 178 K) | ||
| Hazards | |||
| Main hazards | Toxic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
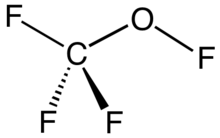
Trifluoromethyl hypofluorite is an organofluorine compound with the formula CF3OF. It exists as a colorless gas at room temperature and is highly toxic.[1] It is a rare example of a hypofluorite (compound with an O−F bond). It can be seen as a similar chemical compound to methanol where every hydrogen atom is replaced by a fluorine atom. It is prepared by the reaction of fluorine gas with carbon monoxide:
- 2 F2 + CO → CF3OF
The gas hydrolyzes only slowly at neutral pH.
Use in organic chemistry
The compound is a source of electrophilic fluorine. It has been used for the preparation of α-fluoroketones from silyl enol ethers.[2] Behaving like a pseudohalogen, it adds to ethylene to give the ether:
- CF3OF + CH2CH2 → CF3OCH2CH2F
References
- ↑ Cady, G (1966). "Trifluoromethyl Hypofluorite". Inorganic Syntheses 8: 168. doi:10.1002/9780470132395.ch43.
- ↑ Middleton, W. J.; Bingham, E. M. (1980). "α-Fluorination of carbonyl compounds with trifluoromethyl hypofluorite". Journal of the American Chemical Society 102: 4845–6. doi:10.1021/ja00534a053.
 |