Chemistry:Trifluorosilane
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trifluorosilane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
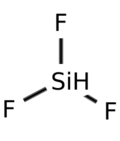
| HF3Si | |||
| Molar mass | 86.09 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 1.86 g/cm3 | ||
| Melting point | −131 °C (−204 °F; 142 K) | ||
| Boiling point | −97.5 °C (−143.5 °F; 175.7 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trifluorosilane is the chemical compound with the formula F3HSi. At standard temperature and pressure, trifluorosilane is a colorless gas.[1] Note that the free radical F3Si is often also referred to as trifluorosilane, although more properly referred to as trifluorosilyl.
Preparation
Trifluorosilane has been purified and separated by low-temperature high-vacuum distillation. One preparation method involves products of the reaction between SbF3 and HSiCl3.[2] HSiCl3 is obtained by copper catalyzed reaction between HCl and Silicon at 200-400 °C.
Formation has also been reported in certain etching operations of silicon.[3]
Properties
The electric dipole moment of trifluorosilane is 1.26 debye.[4] The length of the silicon to fluorine bond is 1.555 Å, Si-H length is 1.55 Å, and ∠FSiF is 110°.[5]
References
- ↑ Perry, Dale L. (2011-06-15). Handbook of Inorganic Compounds, Second Edition. CRC Press. ISBN 9781439814628.
- ↑ Zuckerman, J. J. (2009-09-17). Inorganic Reactions and Methods, The Formation of Bonds to Halogens. John Wiley & Sons. ISBN 9780470145388.
- ↑ Lippold, Marcus; Böhme, Uwe; Gondek, Christoph; Kronstein, Martin; Patzig-Klein, Sebastian; Weser, Martin; Kroke, Edwin (December 2012). "Etching Silicon with HF-HNO 3 -H 2 SO 4 /H 2 O Mixtures - Unprecedented Formation of Trifluorosilane, Hexafluorodisiloxane, and Si-F Surface Groups". European Journal of Inorganic Chemistry 2012 (34): 5714–5721. doi:10.1002/ejic.201200674. ISSN 1434-1948. http://doi.wiley.com/10.1002/ejic.201200674. Retrieved 2014-08-27.
- ↑ Ghosh, S. N.; Trambarulo, Ralph; Gordy, Walter (February 1953). "Electric Dipole Moments of Several Molecules from the Stark Effect". The Journal of Chemical Physics 21 (2): 308–310. doi:10.1063/1.1698877. Bibcode: 1953JChPh..21..308G.
- ↑ Sheridan, John; Gordy, Walter (1 March 1950). "Microwave Spectra and Molecular Constants of Trifluorosilane Derivatives. Si F 3 H, Si F 3 C H 3 , Si F 3 Cl, and Si F 3 Br". Physical Review 77 (5): 719. doi:10.1103/PhysRev.77.719. Bibcode: 1950PhRv...77..719S.
Further reading
- Demaison, J.; Margulès, L.; Breidung, J.; Thiel, W.; Bürger, H. (10 November 1999). "Ab initio anharmonic force field, spectroscopic parameters and equilibrium structure of trifluorosilane". Molecular Physics 97 (9): 1053–1067. doi:10.1080/00268979909482906. Bibcode: 1999MolPh..97.1053D.
 |