Chemistry:Tropacocaine
From HandWiki
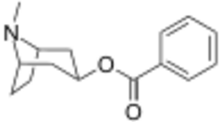
| |
| Names | |
|---|---|
| Systematic IUPAC name
3-exo-8-Methyl-8-azabicyclo[3.2.1]octan-3-yl benzoate | |
| Other names
3β-Benzoyloxytropane
Benzoylpseudotropine | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | tropacocaine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H19NO2 | |
| Molar mass | 245.322 g·mol−1 |
| log P | 2.607 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tropacocaine (tropacaine, benzoylpseudotropine, pseudotropine benzoate, descarbomethoxycocaine) is a cocaine-related alkaloid[1] and a contaminant of street cocaine.[2]
Chemistry
Synthesis
It can be synthesized from tropine using the Mitsunobu reaction.[3]
See also
- Hydroxytropacocaine
- Benzoylecgonine
- p-Fluorotropacocaine
- Indatraline
- Tropane alkaloid
References
- ↑ Jowett, H. A. D.; Pyman, F. L. (1909). "CXVI.?Relation between chemical constitution and physiological action in the tropeines. Part II". Journal of the Chemical Society, Transactions 95: 1020–1032. doi:10.1039/CT9099501020.
- ↑ "Effects of benzoyltropine and tropacocaine on several cholinergic processes in the rat brain". The Journal of Pharmacology and Experimental Therapeutics 254 (2): 584–90. August 1990. PMID 1974643. https://pubmed.ncbi.nlm.nih.gov/1974643/. Retrieved 2021-01-14.
- ↑ [2008-09-10_Cocaine_analog_in_two_steps_from_native_plant_material.shtml "Cocaine analog in two steps from native plant material"]. https://www.seanmichaelragan.com/html/[2008-09-10]_Cocaine_analog_in_two_steps_from_native_plant_material.shtml.
 |
