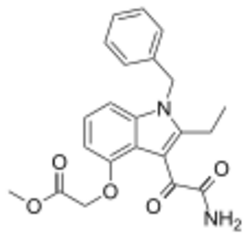
Chemistry:Varespladib methyl
 | |
| Clinical data | |
|---|---|
| Other names | A-002 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H22N2O5 |
| Molar mass | 394.4 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Varespladib methyl (also known as A-002, formerly LY333013 and S-3013) is a secretory phospholipase A2 (sPLA2) inhibitor formerly under development by Anthera Pharmaceuticals as a treatment for acute coronary syndrome (ACS).[1] Varespladib methyl is an orally bioavailable prodrug of the molecule varespladib.[2] From 2006 to 2012, varespladib methyl was under active investigation by Anthera Pharmaceuticals as a potential therapy for several inflammatory diseases, including acute coronary syndrome.[3][4] In March 2012, Anthera halted further investigation of varespladib per a recommendation from an independent Data Safety Monitoring Board.[5] Varespladib and varespladib methyl were characterised as effective molecules in neutralization of snakes venoms[6] and are under experimental evaluation.[7]
Mechanism
Prodrug activation
Varespladib methyl, in contrast to varespladib, is orally bioavailable and after absorption from the GI tract, it undergoes rapid ester hydrolysis to the active molecule – varespladib.[8]
sPLA2 inhbition
Increased levels of sPLA2 have been observed in patients with cardiovascular disease, and may lead to both acute and chronic disease manifestations by promoting vascular inflammation. Plasma levels of sPLA2 can predict coronary events in patients who recently suffered an ACS as well as in those with stable coronary artery disease.[9][10]
Furthermore, sPLA2 remodels lipoproteins, notably low-density lipoproteins (LDL) and their receptors, which are responsible for removing cholesterol from the body. This remodeling can lead to increased deposition of LDL and cholesterol in the artery wall. In combination with chronic vascular inflammation, these deposits lead to atherosclerosis.[11]
Varespladib inhibits the IIA, V and X isoforms of sPLA2 to reduce inflammation, lower and modulate lipid levels, and reduce levels of C-reactive protein (CRP) and interleukin-6 (IL-6), both indicators of inflammation.[1][12]
Snake venom antidote activity
sPLA2 is also present in snake venoms and implicated in their toxicity. It plays a role in the morbidity and mortality from snakebite envenomations, triggering induced cell lysis, disrupted hemostasis, and diminished oxygen transport, as well as myotoxicity and neurotoxicity which can lead to paralysis.[6]
Varespladib methyl, as well as varespladib, were found to be inhibitors of the sPLA2 of snake venoms. Varespladib methyl was less potent than varespladib. Both showed activity against a broad spectrum of different snake venoms originating from six continents.[13] They protected rodents against neurotoxicity and hemostatic toxicity, increasing survival of envenomed animals.[14][7]
Varespladib also effectively inhibited in vitro and in vivo the non-enzymatic myotoxic activity of snake venom's PLA2-like protein (MjTX-II). Co-crystallization of varespladib with MjTX-II toxin (PDB code: 6PWH[15]) revealed that the drug binds to a hydrophobic channel of the protein. This blocks fatty acids from binding there, thus inhibiting their allosteric activation of the toxin, thereby impairing its ability to disrupt cell membranes.[16]
History
Varespladib methyl was originally developed jointly by Eli Lilly and Company and Shionogi & Co., Ltd., and was acquired by Anthera Pharmaceuticals in 2006.[17]
A Phase II study demonstrated selective sPLA2 inhibition as well as statistically significant anti-inflammatory responses and reductions in LDL cholesterol levels.[18] Two other Phase II trials, conducted in patients with coronary artery disease, found significant decreases in sPLA2 and LDL cholesterol levels, as well as C-reactive protein (CRP) and other inflammatory biomarkers.[12][19][20] Varespladib methyl has also been shown to further reduce LDL and inflammatory biomarker levels when administered in conjunction with a cholesterol lowering statin therapy.[21]
In 2010, a Phase III study entitled VISTA-16 was initiated to evaluate the safety and efficacy of short-term treatment with varespladib methyl in subjects with ACS.[22] The trial was halted in March 2012 due to insufficient efficacy.[23] On November 18, 2013, an excess of myocardial infarctions, and of the composite endpoint of cardiovascular mortality, myocardial infarctions and stroke in the VISTA-16 study were reported.[24]
First report on its efficacy as an antidote for snake venoms comes from 2016.[13] Due to oral bioavailability it is considered as a potential first-line field-treatment for snakebite envenomation, which could be applied before provision of definitive medical care.[25]
References
- ↑ 1.0 1.1 "A-002: Short Term (16 week) Treatment of Acute Coronary Syndrome". Anthera Pharmaceuticals, Inc.. http://www.anthera.com/products_a002.asp/.
- ↑ "Varespladib (A-002), a secretory phospholipase A2 inhibitor, reduces atherosclerosis and aneurysm formation in ApoE-/- mice". Journal of Cardiovascular Pharmacology 53 (1): 60–5. January 2009. doi:10.1097/FJC.0b013e318195bfbc. PMID 19129734.
- ↑ "Anthera Licenses Portfolio of Anti-Inflammatory Products From Eli Lilly and Company and Shionogi & Co., Ltd" (Press release). Anthera Pharmaceuticals, Inc. 6 September 2006.
- ↑ "Science: sPLA2". Anthera Pharmaceuticals. http://www.anthera.com/science_spla2.asp.
- ↑ "Anthera Halts VISTA-16 Clinical Study Due to Lack of Efficacy Following Recommendation by the Independent Data Safety Monitoring Board" (Press release). Anthera Pharmaceuticals, Inc. 9 March 2012.
- ↑ 6.0 6.1 "2, a Promising Target for Broad-Spectrum Antivenom Drug Development". BioMed Research International 2017: 6592820. 2017. doi:10.1155/2017/6592820. PMID 29318152.
- ↑ 7.0 7.1 "Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms". Toxins 12 (2): 131. February 2020. doi:10.3390/toxins12020131. PMID 32093386.
- ↑ "Varespladib methyl, an oral phospholipase A2 inhibitor for the potential treatment of coronary artery disease". IDrugs 12 (9): 585–92. September 2009. PMID 19697278.
- ↑ "Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes". Journal of the American College of Cardiology 46 (7): 1249–57. October 2005. doi:10.1016/j.jacc.2005.06.056. PMID 16198839. http://content.onlinejacc.org/cgi/content/full/46/7/1249.
- ↑ "Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease". Circulation 100 (12): 1280–4. September 1999. doi:10.1161/01.CIR.100.12.1280. PMID 10491371.
- ↑ "Lipoprotein-associated and secreted phospholipases A₂ in cardiovascular disease: roles as biological effectors and biomarkers". Circulation 122 (21): 2183–200. November 2010. doi:10.1161/CIRCULATIONAHA.110.936393. PMID 21098459.
- ↑ 12.0 12.1 "Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease". European Heart Journal 32 (8): 999–1005. April 2011. doi:10.1093/eurheartj/ehq374. PMID 21081550. http://eurheartj.oxfordjournals.org/content/early/2011/02/22/eurheartj.ehq374.abstract.
- ↑ 13.0 13.1 "Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation". Toxins 8 (9): 248. August 2016. doi:10.3390/toxins8090248. PMID 27571102.
- ↑ "Micrurus fulvius (Eastern Coral Snake) Venom". Toxins 10 (11): 479. November 2018. doi:10.3390/toxins10110479. PMID 30453607.
- ↑ Bank, RCSB Protein Data. "RCSB PDB - 6PWH: Cystal structure of Myotoxin II from Bothrops moojeni co-crystallized with Varespladib (LY315920)" (in en-US). https://www.rcsb.org/structure/6PWH.
- ↑ Salvador, Guilherme H. M.; Gomes, Antoniel A. S.; Bryan-Quirós, Wendy; Fernández, Julián; Lewin, Matthew R.; Gutiérrez, José María; Lomonte, Bruno; Fontes, Marcos R. M. (2019-11-20). "Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920)". Scientific Reports 9 (1): 17203. doi:10.1038/s41598-019-53755-5. ISSN 2045-2322. PMID 31748642.
- ↑ "Anthera Licenses Portfolio of Anti-Inflammatory Products From Eli Lilly and Company and Shionogi & Co., Ltd" (Press release). Anthera Pharmaceuticals, Inc. 6 September 2006.
- ↑ "Anthera's Varespladib Meets Primary Endpoint In Phase 2 Francis Trial For The Treatment Of Acute Coronary Syndrome" (Press release). Anthera Pharmaceuticals, Inc. 6 May 2009.
- ↑ "Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial". Lancet 373 (9664): 649–58. February 2009. doi:10.1016/S0140-6736(09)60403-7. PMID 19231633. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2809%2960403-7/fulltext.
- ↑ "Varespladib methyl in cardiovascular disease". Expert Opinion on Investigational Drugs 19 (10): 1245–55. October 2010. doi:10.1517/13543784.2010.517193. PMID 20809869.
- ↑ "Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients". Journal of the American College of Cardiology 56 (14): 1079–88. September 2010. doi:10.1016/j.jacc.2010.06.015. PMID 20863951. http://content.onlinejacc.org/cgi/content/short/56/14/1079.
- ↑ "Anthera Enrolls First Patients in Pivotal Varespladib Phase 3 Clinical Study" (Press release). Anthera Pharmaceuticals, Inc. 23 June 2010.
- ↑ Clinical trial number NCT01130246 for "VISTA-16 Trial: Evaluation of Safety and Efficacy of Short-term A-002 Treatment in Subjects With Acute Coronary Syndrome." at ClinicalTrials.gov
- ↑ "Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial". JAMA 311 (3): 252–62. January 2014. doi:10.1001/jama.2013.282836. PMID 24247616.
- ↑ Williams, David J.; Faiz, Mohd Abul; Abela-Ridder, Bernadette; Ainsworth, Stuart; Bulfone, Tommaso C.; Nickerson, Andrea D.; Habib, Abdulrazaq G.; Junghanss, Thomas et al. (2019-02-21). "Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming". PLOS Neglected Tropical Diseases 13 (2): e0007059. doi:10.1371/journal.pntd.0007059. ISSN 1935-2735. PMID 30789906.
External links

