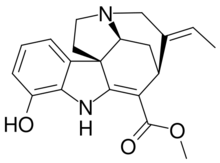
Chemistry:Vinervine
| |
| Names | |
|---|---|
| IUPAC name
Methyl (19E)-12-hydroxy-2,16-didehydrocur-19-en-17-oate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H39NO7 | |
| Molar mass | 453.576 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.
History
The alkaloids are a large group of natural products which are classified according to the part-structure which members of a particular group contain. Vinervine is a monoterpene indole alkaloid of the Vinca sub-group which shares a common biosynthesis with other members, namely that they are derived from strictosidine.[1][2] It was first characterised in 1964[3] and the structures of closely related materials including akuammicine were confirmed in 1983.[4]
Natural occurrence
Vinervine is found in a variety of plants of the Apocynaceae family, including Vinca erecta,[3][5][6] Tabernaemontana divaricata[7][8] and several other flowering plants species that are native to Africa, Asia, and Europe.
Biosynthesis
As with other indole alkaloids, the biosynthesis of vinervine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration.[1]
Research
Plant metabolites have long been studied for their biological activity and alkaloids in particular are major subjects for ethnobotanical research.[9] However, vinervine has had little reported utility.[8][10][11]
See also
References
- ↑ Jump up to: 1.0 1.1 Dewick, Paul M (2002). Medicinal Natural Products. A Biosynthetic Approach. Second Edition. Wiley. pp. 350–359. ISBN 0-471-49640-5. https://books.google.com/books?id=A4zptjOJfKQC&pg=PP1.
- ↑ Saxton, J. E. (1984). "Recent progress in the chemistry of indole alkaloids and mould metabolites". Natural Product Reports 1: 21. doi:10.1039/NP9840100021.
- ↑ Jump up to: 3.0 3.1 "Pseudokopsinine—a new alkaloid from aerial parts of Vinca erecta" (in ru). Doklady Akademii Nauk SSSR 21 (2): 29–31. 1964.
- ↑ Yagudaev, M. R. (1983). "NMR investigation of alkaloids. IV. 13C NMR spectra and structures of norfluorocurarine, akuammicine, vincanidine, and vinervinine". Chemistry of Natural Compounds 19 (2): 199–201. doi:10.1007/BF00580558.
- ↑ "Structure of vincanidine and vinervine.". Chemistry of Natural Compounds 1 (1): 25–30. January 1965. doi:10.1007/BF00571576.
- ↑ Kuchenkova, M. A.; Yuldashev, P. Kh.; Yunusov, S. Yu. (1965). "Vinervine — A new alkaloid from the aboveground part of Vinca erecta". Bulletin of the Academy of Sciences, USSR Division of Chemical Science 14 (12): 2119–2121. doi:10.1007/BF00845999.
- ↑ "Indole alkaloids from cell suspension cultures of Tabernaemontana divaricata and Tabernanthe iboga". Plant Cell Reports 2 (2): 105–7. April 1983. doi:10.1007/BF00270178. PMID 24257961.
- ↑ Jump up to: 8.0 8.1 "Ethnobotany & ethnopharmacology of Tabernaemontana divaricata". The Indian Journal of Medical Research 127 (4): 317–35. April 2008. PMID 18577786.
- ↑ Babiaka, Smith B.; Ntie-Kang, Fidele; Lifongo, Lydia L.; Ndingkokhar, Bakoh; Mbah, James A.; Yong, Joseph N. (2015). "The chemistry and bioactivity of Southern African flora I: A bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes". RSC Advances 5 (54): 43242–43267. doi:10.1039/C5RA01912E. Bibcode: 2015RSCAd...543242B.
- ↑ Ghisalberti, Emilio L.; Pennacchio, Marcello; Alexander, Elizabeth (1998). "Survey of Secondary Plant Metabolites with Cardiovascular Activity". Pharmaceutical Biology 36 (4): 259. doi:10.1076/phbi.36.4.237.4583.
- ↑ Heijden, Robert; Jacobs, Denise; Snoeijer, Wim; Hallard, Didier; Verpoorte, Robert (2004). "The Catharanthus Alkaloids:Pharmacognosy and Biotechnology". Current Medicinal Chemistry 11 (5): 607–628. doi:10.2174/0929867043455846. PMID 15032608.
Further reading
- Edwin Saxton, J. (15 September 2009). Indoles, Part 4: The Monoterpenoid Indole Alkaloids. ISBN 9780470188446. https://books.google.com/books?id=KBmGUvmCOU8C&q=Vinervine&pg=PA1.
 |


