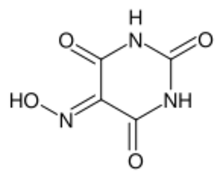
Chemistry:Violuric acid
| |

| |
| Names | |
|---|---|
| IUPAC name
6-Hydroxy-5-nitroso-1H-pyrimidine-2,4-dione
| |
| Other names
2,4,5,6(1H,3H)-Pyrimidinetetrone 5-oxime
5-Hydroxyiminobarbituric acid 5-Isonitrosobarbituric acid Alloxan 5-oxime | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H3N3O4 (anhydrous) C4H3N3O4·H2O (monohydrate) | |
| Molar mass | 157.08 g/mol (anhydrous) 175.10 g/mol (monohydrate) |
| Appearance | Off-white yellow or yellow cream solid |
| Odor | Odorless |
| Melting point | 247 °C (477 °F; 520 K) (decomposes) |
| Boiling point | Decomposes |
| 0.704 g/100 mL (20 °C)[1] | |
| Solubility | Soluble in alcohols |
| Vapor pressure | ~0 mmHg |
| Acidity (pKa) | 4.7 |
| Hazards | |
| Main hazards | Irritant |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Related compounds | |
Related compounds
|
Barbituric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Violuric acid is an organic compound with the formula HON=C(CONH)2CO. It crystallizes as white or off-white monohydrate. The compound has attracted attention because its salts are deeply colored.[2]
Reactions
It readily deprotonated to give salts of the anion [ON=C(CONH)2CO]−, which are often deeply colored.[3][4]
Preparation
It was prepared by Adolf Baeyer by reaction of barbituric acid with nitrous acid.[5] It can also be produced by condensation of alloxan with hydroxylamine.[4][6] as typical for forming the oxime of other carbonyl compounds.
Analytical reagents

Violuric acid and many of its derivatives, such as thiovioluric acid, 1,3-dimethylvioluric acid, and diphenylthiovioluric acid, have historically been used as analytical reagents for spectrophotometric determination and titration of various metals and metal-ions.[8] It was also used as a novel staining/spraying agent for inorganic paper chromatography to identify and separate metals based on color.[9] Most derivatives of violuric acid will also typically form brightly colored salts with most metals and nitrogen bases.[10]
Because of the characteristic and diverse colors that violuric acid forms with alkali metals, it has been used photometrically to determine the amount of sodium in blood serum.[11]
References
- ↑ "Registration Dossier - ECHA". https://echa.europa.eu/registration-dossier/-/registered-dossier/26979/4/9.
- ↑ Guille, Kathy; Harrington, Ross W.; Clegg, William (2007). "Violuric acid monohydrate: A second polymorph with more extensive hydrogen bonding". Acta Crystallographica Section C Crystal Structure Communications 63 (6): o327–o329. doi:10.1107/S010827010701743X. PMID 17551193. Bibcode: 2007AcCrC..63O.327G.
- ↑ Nichol, Gary S.; Clegg, William (2007). "Ammonium Violurate: A Compact Structure with Extensive Hydrogen Bonding in Three Dimensions". Acta Crystallographica Section C Crystal Structure Communications 63 (10): o609–o612. doi:10.1107/S0108270107044241. PMID 17917236. Bibcode: 2007AcCrC..63O.609N.
- ↑ 4.0 4.1 Liebing, Phil; Stein, Franziska; Hilfert, Liane; Lorenz, Volker; Oliynyk, Karyna; Edelmann, Frank T. (2019). "Synthesis and Structural Investigation of Brightly Colored Organoammonium Violurates". Zeitschrift für Anorganische und Allgemeine Chemie 645: 36–43. doi:10.1002/zaac.201800439.
- ↑ Baeyer, Adolf (1863). "Untersuchungen über die Harnsäuregruppe". Annalen der Chemie und Pharmacie 127 (2): 199–236. doi:10.1002/jlac.18631270214. https://zenodo.org/record/1427229.
- ↑ Guinchard, J. (1899). "Ueber die farbigen Salze aus Violursäure und anderen ringförmigen Oximidoketonen". Berichte der Deutschen Chemischen Gesellschaft 32 (2): 1723–1741. doi:10.1002/cber.18990320260.
- ↑ Raston, Colin L.; White, Allan H. (1976). "Salts of the Tris(violurato)ferrate(II) Ion: Crystal Structure of Ammonium Tris(violurato)ferrate(II) Hydrate". Journal of the Chemical Society, Dalton Transactions (19): 1915. doi:10.1039/DT9760001915.
- ↑ Taylor, M. E.; Robinson, R. J. (1962-04-01). "The Use of 1,3-Dimethylvioluric Acid in Spectrophotometric Titrations of Alkali and Alkaline Earth Salts" (in en). Analytical Chemistry 34 (4): 533–536. doi:10.1021/ac60184a026. ISSN 0003-2700.
- ↑ Singh, A. K.; Katyal, Mohan; Singh, R. P. (1976). "Violuric Acids as Analytical Reagents". Current Science 45 (11): 405–408. ISSN 0011-3891.
- ↑ Lorenz, Volker; Liebing, Phil; Engelhardt, Felix; Stein, Franziska; Kühling, Marcel; Schröder, Lea; Edelmann, Frank T. (2019-01-02). "Review: the multicolored coordination chemistry of violurate anions". Journal of Coordination Chemistry 72 (1): 1–34. doi:10.1080/00958972.2018.1560431. ISSN 0095-8972.
- ↑ Muraca, R.F. (1955). "Photometric determination of sodium in blood serum with violuric acid". Chemist Analyst 44: 38–42. https://www.cabdirect.org/cabdirect/abstract/19551404623.
 |

