Earth:Stratospheric aerosol injection (climate engineering)
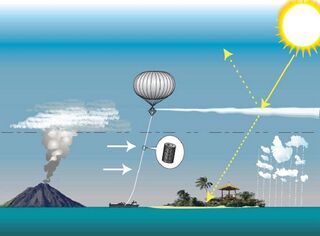
The ability of stratospheric sulfate aerosols to create a global dimming effect has made them a possible candidate for use in solar radiation management climate engineering projects[1] to limit the effect and impact of climate change due to rising levels of greenhouse gases.[2] Delivery of precursor sulfide gases such as sulfuric acid,[3] hydrogen sulfide (H2S) or sulfur dioxide (SO2) by artillery, aircraft[4] and balloons has been proposed.[5] It presently appears that this proposed method could counter most climatic changes, take effect rapidly, have very low direct implementation costs, and be reversible in its direct climatic effects.
One study calculated the impact of injecting sulfate particles, or aerosols, every one to four years into the stratosphere in amounts equal to those lofted by the volcanic eruption of Mount Pinatubo in 1991,[6] but did not address the many technical and political challenges involved in potential solar radiation management (SRM) efforts.[7] If found to be economically, environmentally and technologically viable, such injections could provide a "grace period" of up to 20 years before major cutbacks in greenhouse gas emissions would be required, the study concludes.
It has been suggested that the direct delivery of precursors could be achieved using sulfide gases such as dimethyl sulfide, sulfur dioxide (SO2), carbonyl sulfide, or hydrogen sulfide (H2S).[5] These compounds would be delivered using artillery, aircraft (such as the high-flying F-15C)[4] or balloons, and result in the formation of compounds with the sulfate anion SO42−.[5]
According to estimates, "one kilogram of well placed sulfur in the stratosphere would roughly offset the warming effect of several hundred thousand kilograms of carbon dioxide."[8]
Arguments for the technique
The arguments in favour of this approach in comparison to other possible means of solar radiation management are:
- Mimics a natural process:[9] Stratospheric sulfur aerosols are created by existing natural processes (especially volcanoes), whose impacts have been studied via observations.[10] This contrasts with other, more speculative solar radiation management techniques which do not have natural analogs (e.g., space sunshade).
- Technological feasibility: In contrast to other proposed solar radiation management techniques, such as marine cloud brightening and space sunshades, much of the required technology is pre-existing: chemical manufacturing, artillery shells, high-altitude aircraft, weather balloons, etc.[5]
- Cost: The low-tech nature of this approach has led commentators to suggest it would cost less than many other interventions. Costs cannot be derived in a wholly objective fashion, as pricing can only be roughly estimated at an early stage. However, many sources suggest that it would be cheap relative to cutting emissions.[11][12][13] The annual cost of delivering 5 million tons of an albedo enhancing aerosol to an altitude of 20 to 30 km is estimated at 2 to 8 billion USD.[14] Around 5 million tons of SO2 delivered annually is predicted to sufficiently offset the expected warming over the next century.[14] SO2 can be purchased online for as little as 500 USD per ton.[15] In comparison, the annual cost estimates for climate damage or emission mitigation range from 200 billion USD to 2 trillion USD.[14]
- Efficacy: Most proposed solar radiation management techniques can only provide a limited intervention in the climate—one cannot reduce the temperature by more than a certain amount with each technique. New research by Lenton and Vaughan suggests that this technique may have a high radiative 'forcing potential'.[16]
Efficacy issues
It is uncertain how effective any solar radiation management technique would be, due to the difficulties modeling their impacts and the complex nature of the global climate system. Certain efficacy issues are specific to stratospheric aerosols.
- Lifespan of aerosols: Tropospheric sulfur aerosols are short lived.[17] Delivery of particles into the lower stratosphere in the arctic will typically ensure that they remain aloft only for a few weeks or months, as air in this region is predominantly descending.[18] To ensure endurance, higher-altitude delivery is needed, ensuring a typical endurance of several years by enabling injection into the rising leg of the Brewer-Dobson circulation above the tropical tropopause. Further, sizing of particles is crucial to their endurance.[19]
- Aerosol delivery: There are two proposals for how to create a stratospheric sulfate aerosol cloud, either through release of a precursor gas (SO2) or the direct release of sulfuric acid (H2SO4) and these face different challenges.[20] If SO2 gas is released it will oxidise to form H2SO4 and then condense to form droplets far from the injection site.[21] Releasing SO2 would not allow control over the size of the particles that are formed but would not require a sophisticated release mechanism. Simulations suggest that as the SO2 release rate is increased there would be diminishing returns on the cooling effect, as larger particles would be formed which have a shorter lifetime and are less effective scatterers of light.[22] If H2SO4 is released directly then the aerosol particles would form very quickly and in principle the particle size could be controlled although the engineering requirements for this are uncertain. As direct H2SO4 release would allow control over the particle size it could be possible to avoid some of the inefficiencies associated with SO2 release.[20]
Possible side effects
Climate engineering and solar radiation management in general are controversial, and pose various problems and risks. However, certain problems are specific to, or more pronounced with this particular technique.[23]
- Ozone depletion: is a potential side effect of sulfur aerosols;[24][25] and these concerns have been supported by modelling.[26] However, this may only occur if high enough quantities of aerosols drift to, or are deposited in, Polar stratospheric clouds before the levels of CFCs and other ozone destroying gases fall naturally to safe levels because stratospheric aerosols, together with the ozone destroying gases, are responsible for ozone depletion.[27]
- Whitening of the sky: Stratospheric aerosols have the potential to whiten the sky and cause more colorful sunsets, dependent on the amount being sprayed.[28] According to a study on cleaner air, resulting solar brightening has been responsible for an increase in corn production over the past 30 years.[29]
- Tropopause warming: and the humidification of the stratosphere.[25]
- Regional warming: Based on the results of the 2014-2015 Geoengineering Model Intercomparison Project, a model with a standard stratospheric aerosol injection scenario, temperatures in the tropics would cool, and higher latitudes warm, ice sheet, and Arctic sea ice decline would still continue, albeit at a reduced rate. Extreme temperature anomalies would also still increase, but to a lesser degree. In regards to these model results, the author of the study Alan Robock noted:
If geoengineering were halted all at once, there would be rapid temperature and precipitation increases at 5–10 times the rates from gradual global warming.[30]
- Stratospheric temperature change: Aerosols can also absorb some radiation from the Sun, the Earth and the surrounding atmosphere. This changes the surrounding air temperature and could potentially impact on the stratospheric circulation, which in turn may impact the surface circulation.[31]
- Regional hydrologic responses: Based on the results of the Geoengineering Model Intercomparison Project, there would be a reduction in the global average precipitation around the world, particularly in summer monsoon regions.[30]
- Health effects: Whilst the sulfate particles are natural, they still have potential health effects.[32] Minimising these effects is principally achieved by ensuring the particles stay aloft as long as possible, thus reducing the tonnages returning into the lower atmosphere.
Aerosol formation
Primary aerosol formation, also known as homogeneous aerosol formation, results when gaseous SO2 combines with oxygen and water to form aqueous sulfuric acid (H2SO4). This acidic liquid solution is in the form of a vapor and condenses onto particles of solid matter, either meteoritic in origin or from dust carried from the surface to the stratosphere. Secondary or heterogeneous aerosol formation occurs when H2SO4 vapor condenses onto existing aerosol particles. Existing aerosol particles or droplets also run into each other, creating larger particles or droplets in a process known as coagulation. Warmer atmospheric temperatures also lead to larger particles. These larger particles would be less effective at scattering sunlight because the peak light scattering is achieved by particles with a diameter of 0.3 μm.[33]
Methods
Various techniques have been proposed for delivering the aerosol precursor gases (H2S and SO2).[2] The required altitude to enter the stratosphere is the height of the tropopause, which varies from 11 kilometres (6.8 mi/36,000 ft) at the poles to 17 kilometres (11 mi/58,000 ft) at the equator.
- Airliners could use lower-quality sulfur-rich fuels on higher altitudes. That approach would utilize regular flights and enable airlines to use cheaper fuels on long-distance flights. It would require using separate fuel tanks for takeoff and landing in populated areas, due to toxicity and olfactory sensations of sulfur oxides. This can be achieved in many airliners without difficulty, since they already have separate and selectable wing and fuselage fuel tanks.
- Civilian aircraft including the Boeing 747-400 and Gulfstream G550/650, C-37A could be modified at relatively low cost to deliver sufficient amounts of required material.[34]
- Military aircraft such as the F15-C variant of the F-15 Eagle have the necessary flight ceiling, but limited payload. Military tanker aircraft such as the KC-135 Stratotanker and KC-10 Extender also have the necessary ceiling and have greater payload capacity.[4]
- Modified artillery might have the necessary capability,[35] but requires a polluting and expensive gunpowder charge to loft the payload. Railgun artillery could be a non-polluting alternative.
- High-altitude balloons can be used to lift precursor gases, in tanks, bladders or in the balloons' envelope. Balloons can also be used to lift pipes and hoses, but no moored balloon has ever been deployed to the necessary altitude.[citation needed]
Material options
Precursor gases such as sulfur dioxide and hydrogen sulfide have been considered. Use of gaseous sulfuric acid appears to reduce the problem of aerosol growth.[3] Materials such as photophoretic particles, titanium dioxide, and diamond are also under consideration.[36][37][38]
Injection system
The latitude and distribution of injection locations has been discussed by various authors. Whilst a near-equatorial injection regime will allow particles to enter the rising leg of the Brewer-Dobson circulation, several studies have concluded that a broader, and higher-latitude, injection regime will reduce injection mass flow rates and/or yield climatic benefits.[39][40] Concentration of precursor injection in a single longitude appears to be beneficial, with condensation onto existing particles reduced, giving better control of the size distribution of aerosols resulting.[41] The long residence time of carbon dioxide in the atmosphere may require a millennium-timescale commitment to SRM[42] if aggressive emissions abatement is not pursued simultaneously.
Outdoors research
Almost all work to date on stratospheric sulfate injection has been limited to modelling and laboratory work. A Russian team tested aerosol formation in the lower troposphere using helicopters.[43] The Stratospheric Particle Injection for Climate Engineering (SPICE) project planned on a limited field test in order to evaluate a potential delivery system, but this component of the project was canceled. In 2015, a group based at Harvard University has described a potential field experiment to test the possible damage to stratospheric ozone from stratospheric sulfate injection,[44] and a first test is scheduled for 2017/2018, taking place in the Tucson desert.[45]
Governance
Most of the existing governance of stratospheric sulfate aerosols is from that which is applicable to solar radiation management more broadly. However, some existing legal instruments would be relevant to stratospheric sulfate aerosols specifically. At the international level, the Convention on Long-Range Transboundary Air Pollution (CLRTAP Convention) obligates those countries which have ratified it to reduce their emissions of particular transboundary air pollutants. Notably, both solar radiation management and climate change (as well as greenhouse gases) could satisfy the definition of "air pollution" which the signatories commit to reduce, depending on their actual negative effects.[46] Commitments to specific values of the pollutants, including sulfates, are made through protocols to the CLRTAP Convention. Full implementation or large scale climate response field tests of stratospheric sulfate aerosols could cause countries to exceed their limits. However, because stratospheric injections would be spread across the globe instead of concentrated in a few nearby countries, and could lead to net reductions in the "air pollution" which the CLRTAP Convention is to reduce, it is uncertain how the Convention's Implementation Committee and Executive Body would respond to such event.
The stratospheric injection of sulfate aerosols would cause the Vienna Convention for the Protection of the Ozone Layer to be applicable, due to their possible deleterious effects on stratospheric ozone. That treaty generally obligates its Parties to enact policies to control activities which "have or are likely to have adverse effects resulting from modification or likely modification of the ozone layer."[47] The Montreal Protocol to the Vienna Convention prohibits the production of certain ozone depleting substances, via phase outs. Sulfates are presently not among the prohibited substances.
In the United States, the Clean Air Act might give the United States Environmental Protection Agency authority to regulate stratospheric sulfate aerosols, although the agency has not yet taken such action.[48]
See also
- Solar radiation management
- Climate engineering
- Climate change
- Cloud seeding
- Chemtrail conspiracy theory
- Weather Modification Operations and Research Board
References
- ↑ Launder B.; J.M.T. Thompson (2008). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A 366 (1882): 4039–4056. doi:10.1098/rsta.2008.0132. PMID 18757275. Bibcode: 2008RSPTA.366.4039C. http://journals.royalsociety.org/content/84j11614488142u8/.
- ↑ 2.0 2.1 Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change 77 (3–4): 211–220. doi:10.1007/s10584-006-9101-y. http://www.springerlink.com/content/t1vn75m458373h63/fulltext.pdf.
- ↑ 3.0 3.1 Pierce, J. R.; Weisenstein, D. K.; Heckendorn, P.; Peter, T.; Keith, D. W. (2010). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft". Geophysical Research Letters 37 (18): n/a. doi:10.1029/2010GL043975. Bibcode: 2010GeoRL..3718805P.
- ↑ 4.0 4.1 4.2 Robock, A.; Marquardt, A.; Kravitz, B.; Stenchikov, G. (2009). "Benefits, risks, and costs of stratospheric geoengineering". Geophysical Research Letters 36 (19): L19703. doi:10.1029/2009GL039209. Bibcode: 2009GeoRL..3619703R.
- ↑ 5.0 5.1 5.2 5.3 Rasch, P. J.; Tilmes, S.; Turco, R. P.; Robock, A.; Oman, L.; Chen, C.; Stenchikov, G. L.; Garcia, R. R. (Nov 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences 366 (1882): 4007–4037. doi:10.1098/rsta.2008.0131. ISSN 1364-503X. PMID 18757276. Bibcode: 2008RSPTA.366.4007R.
- ↑ Wigley, T. M. L. (Oct 2006). "A combined mitigation/geoengineering approach to climate stabilization". Science 314 (5798): 452–454. doi:10.1126/science.1131728. ISSN 0036-8075. PMID 16973840. Bibcode: 2006Sci...314..452W.
- ↑ "Stratospheric Injections Could Help Cool Earth, Computer Model Shows – News Release". National Center for Atmospheric Research. September 14, 2006. http://www.ucar.edu/news/releases/2006/injections.shtml. Retrieved June 15, 2011.
- ↑ David G. Victor, M. Granger Morgan, Jay Apt, John Steinbruner, and Katharine Ricke (March–April 2009). "The Geoengineering Option:A Last Resort Against Global Warming?". Geoengineering. Council on Foreign Affairs. http://www.cfr.org/publication/18635/. Retrieved August 19, 2009.
- ↑ Bates, S. S.; Lamb, B. K.; Guenther, A.; Dignon, J.; Stoiber, R. E. (1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry 14: 315–337. doi:10.1007/BF00115242. Bibcode: 1992JAtC...14..315B.
- ↑ Zhao, J.; Turco, R. P.; Toon, O. B. (1995). "A model simulation of Pinatubo volcanic aerosols in the stratosphere". Journal of Geophysical Research 100: 7315–7328. doi:10.1029/94JD03325. Bibcode: 1995JGR...100.7315Z.
- ↑ Brahic, Catherine (February 25, 2009). "Hacking the planet: The only climate solution left? (NB cost data in accompanying image)". Reed Business Information Ltd.. https://www.newscientist.com/article/mg20126973.600-hacking-the-planet-the-only-climate-solution-left.html?full=true. Retrieved February 28, 2009.
- ↑ "The Royal Society". https://royalsociety.org/~/media/Royal_Society_Content/policy/publications/2009/8693.pdf. Retrieved 2015-11-18.
- ↑ Council, National Research. Climate Intervention: Reflecting Sunlight to Cool Earth. doi:10.17226/18988. http://www.nap.edu/catalog/18988/climate-intervention-reflecting-sunlight-to-cool-earth.
- ↑ 14.0 14.1 14.2 McClellan, Justin; Keith, David W.; Apt, Jay (2012-01-01). "Cost analysis of stratospheric albedo modification delivery systems" (in en). Environmental Research Letters 7 (3): 034019. doi:10.1088/1748-9326/7/3/034019. ISSN 1748-9326. http://stacks.iop.org/1748-9326/7/i=3/a=034019.
- ↑ "Alibaba Manufacturer Directory - Suppliers, Manufacturers, Exporters & Importers". https://www.alibaba.com/product-detail/liquid-sulfur-dioxide_60203655263.html?spm=a2700.7724857.0.0.uKlsDj&s=p&title=liquid%2520sulfur%2520dioxide.
- ↑ Lenton, Tim; Vaughan. "Radiative forcing potential of climate geoengineering". http://www.atmos-chem-phys-discuss.net/9/2559/2009/acpd-9-2559-2009.pdf. Retrieved February 28, 2009.
- ↑ Monastersky, Richard (1992). "Haze clouds the greenhouse—sulfur pollution slows global warming—includes related article". Science News. http://findarticles.com/p/articles/mi_m1200/is_/ai_12140641.
- ↑ http://www.crosswalk.com/news/11572945/
- ↑ Rasch, P. J.; Crutzen, P. J.; Coleman, D. B. (2008). "Exploring the geoengineering of climate using stratospheric sulfate aerosols: the role of particle size". Geophysical Research Letters 35 (2): L02809. doi:10.1029/2007GL032179. Bibcode: 2008GeoRL..3502809R. https://zenodo.org/record/1038603.
- ↑ 20.0 20.1 Pierce, Jeffrey R.; Weisenstein, Debra K.; Heckendorn, Patricia; Peter, Thomas; Keith, David W. (2010-09-01). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft" (in en). Geophysical Research Letters 37 (18): L18805. doi:10.1029/2010GL043975. ISSN 1944-8007. Bibcode: 2010GeoRL..3718805P. http://onlinelibrary.wiley.com/doi/10.1029/2010GL043975/abstract.
- ↑ Niemeier, U.; Schmidt, H.; Timmreck, C. (2011-04-01). "The dependency of geoengineered sulfate aerosol on the emission strategy" (in en). Atmospheric Science Letters 12 (2): 189–194. doi:10.1002/asl.304. ISSN 1530-261X. http://onlinelibrary.wiley.com/doi/10.1002/asl.304/abstract.
- ↑ "ACP - Peer review - What is the limit of climate engineering by stratospheric injection of SO2?". http://www.atmos-chem-phys.net/15/9129/2015/acp-15-9129-2015-discussion.html.
- ↑ Robock, A. (2008). "20 reasons why geoengineering may be a bad idea". Bulletin of the Atomic Scientists 64 (2): 14–19. doi:10.2968/064002006.
- ↑ Tabazadeh, A.; Drdla, K.; Schoeberl, R.; Hamill, P.; Toon, B. (Mar 2002). "Arctic "ozone hole" in a cold volcanic stratosphere" (Free full text). Proceedings of the National Academy of Sciences of the United States of America 99 (5): 2609–2612. doi:10.1073/pnas.052518199. ISSN 0027-8424. PMID 11854461. PMC 122395. Bibcode: 2002PNAS...99.2609T. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=11854461.
- ↑ 25.0 25.1 http://www.cosis.net/abstracts/EGU2008/10823/EGU2008-A-10823.pdf
- ↑ Heckendorn, P; Weisenstein, D; Fueglistaler, S; Luo, B P; Rozanov, E; Schraner, M; Thomason, L W; Peter, T (2009). "The impact of geoengineering aerosols on stratospheric temperature and ozone". Environmental Research Letters 4 (4): 045108. doi:10.1088/1748-9326/4/4/045108. Bibcode: 2009ERL.....4d5108H.
- ↑ Hargreves, Ben (2010). "Protecting the Planet". Professional Engineering (Professional Engineering Publishing) 23 (19): 18–22. ISSN 0953-6639.
- ↑ Olson, D. W., R. L. Doescher, and M. S. Olson (February 2004). "When the Sky Ran Red: The Story Behind The Scream". Sky & Telescope: 29–35.
- ↑ "A bright sun today? It's down to the atmosphere". The Guardian. 2017. https://www.theguardian.com/news/2017/mar/21/a-bright-sun-today-its-down-to-the-atmosphere.
- ↑ 30.0 30.1 Alan Robock (2015). "Stratospheric Aerosol Geoengineering". Royal Society of Chemistry. doi:10.1063/1.4916181. http://climate.envsci.rutgers.edu/pdf/RobockStratAerosolGeo.pdf.
- ↑ Ferraro, A. J., Highwood, E. J., Charlton-Perez, A. J. (2011). "Stratospheric heating by geoengineering aerosols". Geophysical Research Letters 37 (24): L24706. doi:10.1029/2011GL049761. Bibcode: 2011GeoRL..3824706F.
- ↑ http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4102961
- ↑ Keith, David W. (2010). "Photophoretic Levitation of Engineered Aerosols for Geoengineering". Proceedings of the National Academy of Sciences of the United States of America 107 (38): 16428–16431. doi:10.1073/pnas.1009519107. ISSN 0027-8424. PMID 20823254. Bibcode: 2010PNAS..10716428K.
- ↑ McClellan, Justin; Keith, David; Apt, Jay (30 August 2012). "Cost Analysis of Stratospheric Albedo Modification Delivery Systems". Environmental Research Letters 7 (3): 3 in 1–8. doi:10.1088/1748-9326/7/3/034019. http://iopscience.iop.org/1748-9326/7/3/034019/. Retrieved 23 June 2015.
- ↑ PICATINNY ARSENAL DOVER N J. "PARAMETRIC STUDIES ON USE OF BOOSTED ARTILLERY PROJECTILES FOR HIGH ALTITUDE RESEARCH PROBES, PROJECT HARP,". http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD0601409. Retrieved February 25, 2009.
- ↑ Keith, David W. (2010). "Photophoretic levitation of engineered aerosols for geoengineering". Proc Natl Acad Sci USA 107 (38): 16428–16431. doi:10.1073/pnas.1009519107. PMID 20823254. Bibcode: 2010PNAS..10716428K.
- ↑ Keith, D.W. and D. K. Weisenstein (2015). "Solar geoengineering using solid aerosol in the stratosphere". Atmos. Chem. Phys. Discuss. 15: 11799–11851. doi:10.5194/acpd-15-11799-2015. Bibcode: 2015ACPD...1511799W. http://www.atmos-chem-phys-discuss.net/15/11799/2015/acpd-15-11799-2015.html. Retrieved 8 June 2015.
- ↑ Ferraro, A. J., A. J. Charlton-Perez, E. J. Highwood (2015). "Stratospheric dynamics and midlatitude jets under geoengineering with space mirrors and sulfate and titania aerosols". Journal of Geophysical Research: Atmospheres 120 (2): 414–429. doi:10.1002/2014JD022734. Bibcode: 2015JGRD..120..414F. http://onlinelibrary.wiley.com/doi/10.1002/2014JD022734/abstract. Retrieved 8 June 2015.
- ↑ English, J. M.; Toon, O. B.; Mills, M. J. (2012). "Microphysical simulations of sulfur burdens from stratospheric sulfur geoengineering". Atmospheric Chemistry and Physics 12 (10): 4775–4793. doi:10.5194/acp-12-4775-2012. Bibcode: 2012ACP....12.4775E.
- ↑ MacCracken, M. C.; Shin, H. -J.; Caldeira, K.; Ban-Weiss, G. A. (2012). "Climate response to imposed solar radiation reductions in high latitudes". Earth System Dynamics Discussions 3 (2): 715–757. doi:10.5194/esdd-3-715-2012. Bibcode: 2012ESDD....3..715M.
- ↑ Niemeier, U.; Schmidt, H.; Timmreck, C. (2011). "The dependency of geoengineered sulfate aerosol on the emission strategy". Atmospheric Science Letters 12 (2): 189–194. doi:10.1002/asl.304.
- ↑ Brovkin, V.; Petoukhov, V.; Claussen, M.; Bauer, E.; Archer, D.; Jaeger, C. (2008). "Geoengineering climate by stratospheric sulfur injections: Earth system vulnerability to technological failure". Climatic Change 92 (3–4): 243–259. doi:10.1007/s10584-008-9490-1.
- ↑ Izrael, Yuri (2009). "Field studies of a geo-engineering method of maintaining a modern climate with aerosol particles". Russian Meteorology and Hydrology 34 (10): 635–638. doi:10.3103/S106837390910001X. https://link.springer.com/article/10.3103%2FS106837390910001X. Retrieved 8 June 2015.
- ↑ Dykema, John A. (2014). "Stratospheric controlled perturbation experiment: a small-scale experiment to improve understanding of the risks of solar geoengineering". Phil. Trans. R. Soc. A 372 (2013): 20140059. doi:10.1098/rsta.2014.0059. Bibcode: 2014RSPTA.37240059D. http://rsta.royalsocietypublishing.org/content/372/2031/20140059.short. Retrieved 8 June 2015.
- ↑ "Harvard Scientists Taking Geoengineering Into the Field". ClimateCentral. 2017. http://www.climatecentral.org/news/harvard-scientists-taking-geoengineering-into-the-field-21362.
- ↑ Convention on Long-Range Transboundary Air Pollution art. 1, Nov. 13, 1979, 1302 U.N.T.S. 219, Article 1
- ↑ Vienna Convention for the Protection of the Ozone Layer, opened for signature Mar. 22, 1985, 1513 U.N.T.S. 293, Article 1
- ↑ Hester, Tracy D. (2011). "Remaking the World to Save it: Applying U.S. Environmental Laws to Climate Engineering Projects". Ecology Law Quarterly 38 (4): 876–880. http://scholarship.law.berkeley.edu/elq/vol38/iss4/2/. Retrieved 5 June 2015.
Further reading
- Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change 77 (3–4): 211–220. doi:10.1007/s10584-006-9101-y. http://www.springerlink.com/content/t1vn75m458373h63/fulltext.pdf.
External links
- What can we do about climate change?, Oceanography magazine
- Global Warming and Ice Ages: Prospects for Physics-Based Modulation of Global Change, Lawrence Livermore National Laboratory
- The Geoengineering Option:A Last Resort Against Global Warming?, Council on Foreign Relations
- Geo-Engineering Climate Change with Sulfate Aerosols, Pacific Northwest National Laboratory
- Geo-Engineering Research, Parliamentary Office of Science and Technology
- Geo-engineering Options for Mitigating Climate Change, Department of Energy and Climate Change
- Unilateral Geoengineering, Council on Foreign Relations
- An overview of geoengineering of climate using stratospheric sulphate aerosols, The Royal Society
- US patent 5003186 "Stratospheric Welsbach seeding for reduction of global warming"
